Meningioma genetics research leads to new treatment opportunities
March 26, 2018 Source: Sina medicine Author: unique eye medicine
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Meningioma
Currently, meningiomas are the most common intracranial tumors. It is estimated that the incidence rate is about 7.86 / 100,000. Meningioma is more common in middle-aged and elderly patients, especially after age 65, and its risk increases with age. Statistics on the pathogenesis of meningiomas, 90% in the intracranial, 10% in the spinal cord membrane.
From the point of view of benign and malignant, about 80% of meningiomas are benign, equivalent to grade I in the WHO classification; 20% of meningiomas will progress to malignancy, equivalent to grades II and III in the WHO classification. The vast majority of meningiomas can be cured by surgery, but about 20% of cases will recur and progress to malignant tumors. This part of progressive meningioma has a poor prognosis and urgently requires more precise treatment.
In recent years, thanks to the rapid development of molecular genetics, the mutated genes of various types of meningioma are being gradually identified, which paves the way for the precise treatment of progressive meningioma.
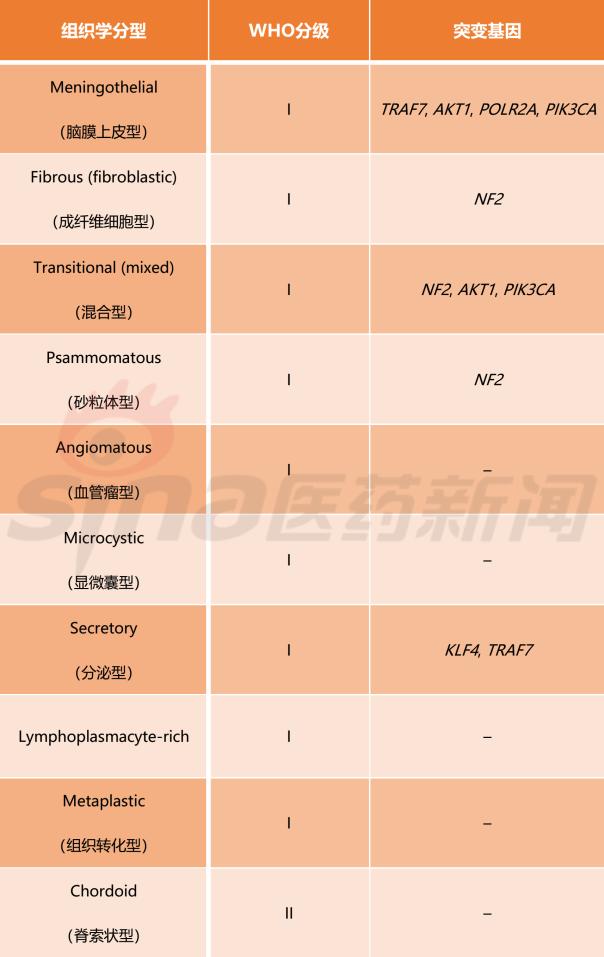
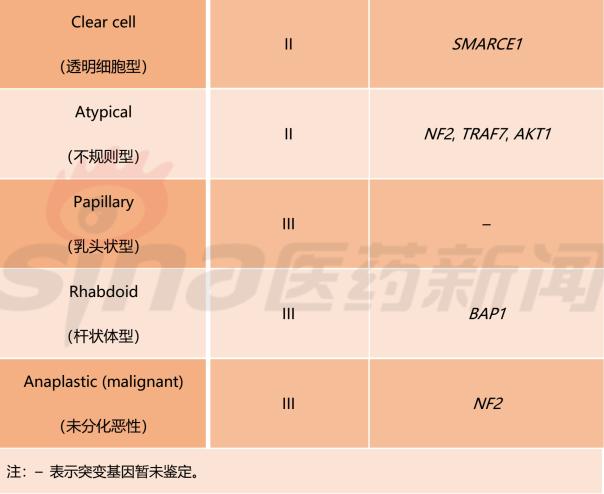
Meningioma WHO classification classification criteria
Table 1 lists the meningiomas classification criteria established by the WHO in 2016. This standard divides meningiomas into 15 subtypes and uses grades I, II, and III to indicate the benign and malignant tumor subtypes. In addition, this standard fully absorbs the recent advances in genetic research on meningioma, and among the 15 meningioma subtypes, 9 subtypes have identified mutant genes.
Table 1 Classification criteria for meningioma classification developed by WHO (2016 version)
Tissue site, gene and target distribution
The study found that the location of meningioma is different, and its genetic molecular characteristics are also very different, and the treatment will change accordingly. For example, Convexity meningiomas commonly have NF2 and SMARCB1 gene mutations; Skull-base meningiomas commonly have AKT1, SMO, KLF4, TRAF7, POLR2A and PIK3CA mutations; Spinal cord meningiomas Common mutations in the SMARCE1 gene, as shown in Figure 1.
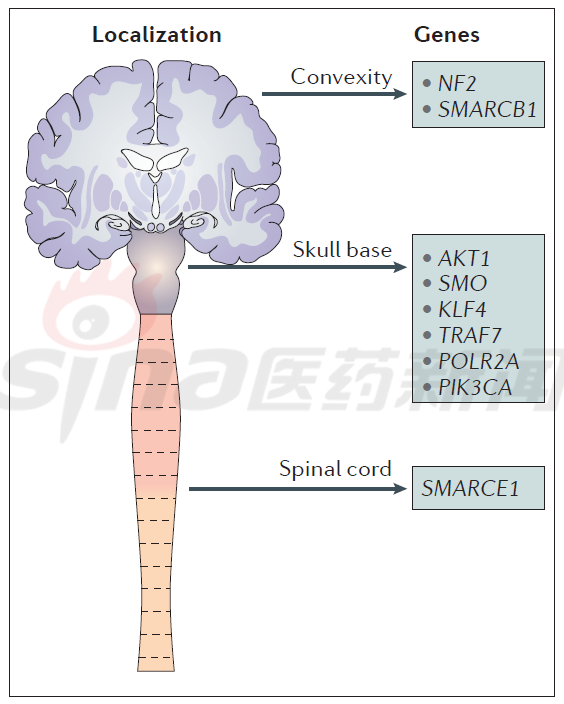
Figure 1 Molecular genetic characteristics of meningiomas at various sites
Most genetic alterations in convex or skull base meningioma drive radically the key mitogenic pathways, including phosphoinositide 3-kinase-AKT, rapamycin target protein (mTOR), and extracellular signal-regulated kinase ( ERK); gene mutations also overexpress tyrosine kinase receptors such as vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR) or insulin-like growth factor receptor (IGFR).
Therefore, for some non-removable, recurrent and/or invasive high-grade meningioma, evaluation of therapeutic drugs, such as tyrosine kinase inhibition, AKT inhibition and mTOR inhibition, is shown in Figure 2. . It is worth mentioning that the direct drug-targeting drug trappectedin has shown good results in in vitro tests and is currently undergoing large-scale clinical trials.
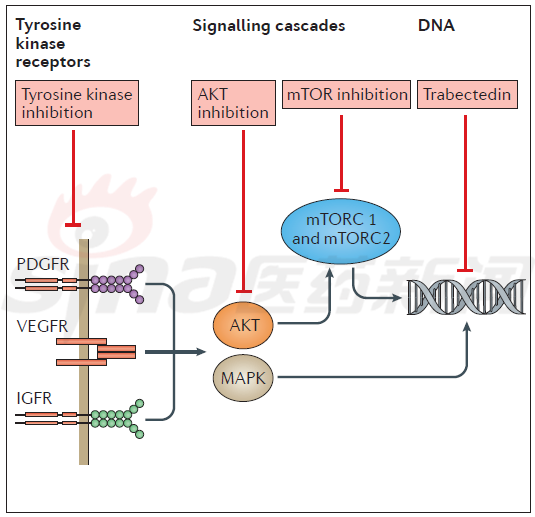
Figure 2 Types of treatment for recurrent meningioma
Meningioma treatment
Many cellular signaling pathways have been identified as part of the growth of meningiomas, including mitogen-activated protein kinase (MAPK) channels, phosphoinositide 3-kinase-AKT, and rapamycin target protein (mTOR). Channel and Hedgehog channel. Figure 3 outlines the current treatment options for meningioma, encompassing a variety of molecular targets. Select the appropriate treatment based on the patient's own biomarker. As shown in table 2.
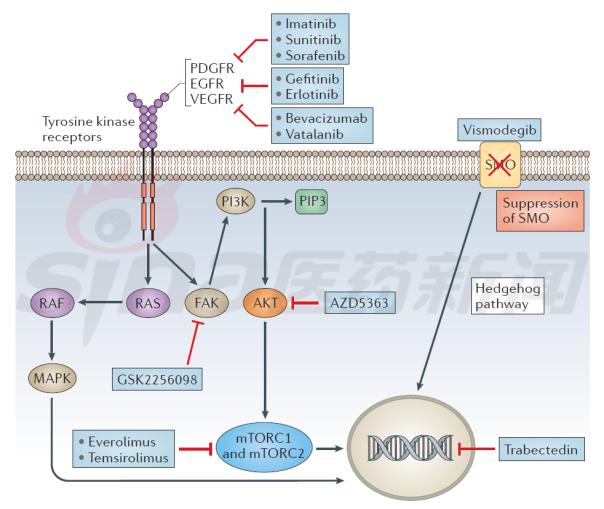
Figure 3 Overview of meningioma activation signaling pathways and targeted drugs
Table 2 Meningiomas candidate targeted drugs
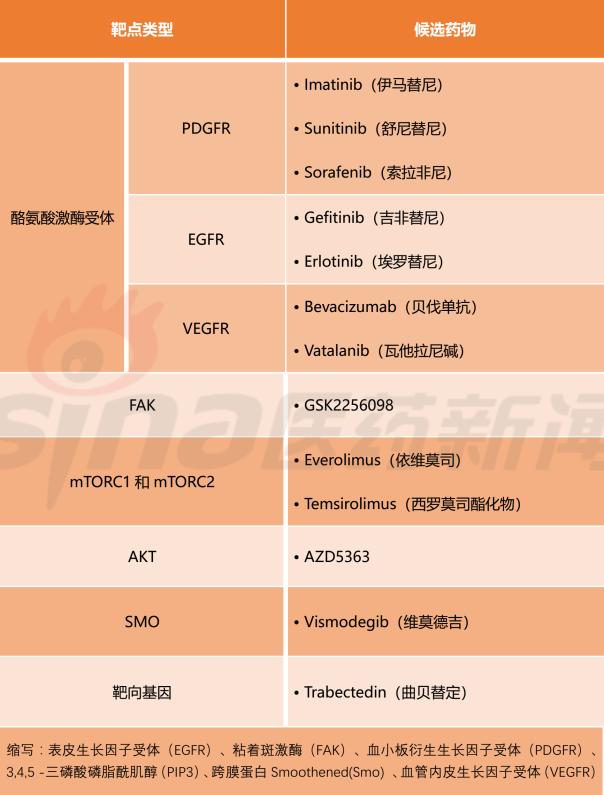
Conclusion
Meningioma is the most common intracranial tumor. In addition to the common mutant gene NF2, mutations such as SMO, AKT1, TRAF7, KLF4, PIK3CA and POLR2A have been discovered in the past two years. Mutations in the SMO, AKT1 and TERT promoters increase the risk of disease recurrence and can be used as a poor prognosis. index. Methylation-based meningioma classification is a promising approach that allows different types of patients to receive appropriate treatment and monitoring. The new mouse model supports the role of NF2 and other genes in tumor progression.
Although the treatment of invasive and/or recurrent meningioma is primarily focused on surgery and/or radiation therapy, these new gene discoveries provide an alternative approach to medical treatment, and many treatment options are undergoing clinical trials. Therefore, genomics has changed our understanding of the molecular genetics of meningiomas, and ongoing clinical trials have the potential to alter the treatment of meningioma.
references
[1]Yue Yingjie, Fei Wei, Zhang Jian, Zheng Xuejun, Wang Xigao, Guo Feng. Differences in MRI findings of WHO I, II, and III meningiomas [J]. International Journal of Neurology and Neurosurgery, 2012, 39 (02) :138-142.
[2]NatureReviewsNeurology 14, pages106–115 (2018)|doi:10.1038/nrneurol.2017.168
Concealed Hinges,Screw-On Hinges,Concealed Door Hinges,Concealed Cabinet Hinges
Ningbo Hengchieh Locking Technology Co., Ltd. , https://www.yh-lock.com