Release date: 2016-05-27
Founded in December 2009, Kayudi Biotechnology (Beijing) Co., Ltd. is a national high-tech enterprise dedicated to research, development, production and sales of genetic and molecular diagnostic systems. In 2013, Kayudi was selected as “the most promising entrepreneurial enterprise for overseas studentsâ€. In 2015, Kayudi ranked 16th among the top 50 most valuable investment companies.
The molecular diagnostics industry is developing rapidly, and the industry has high technical barriers.
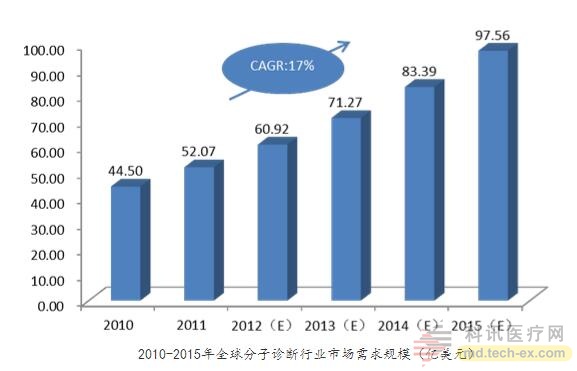
In recent years, molecular diagnostics has been the fastest growing sub-sector in the in vitro diagnostic industry. According to the "China Bio-industry Development Report", in 2010, China's molecular diagnostics accounted for 8.9% of the global in vitro diagnostic products market, about 4.45 billion US dollars, an average annual growth rate of 17% in the past decade. At present, China has issued a number of policies for grading diagnosis and treatment and precision medical treatment to solve the problem of uneven distribution of medical resources and overcrowding of large hospitals. Molecular diagnostic technology has fast, accurate diagnostic features, but it has a high technical threshold.
The advantages of Cayudi products are portable, fast and accurate, and can be used for emergency screening and chronic disease screening.
The main principle of molecular diagnosis is the use of molecular biology methods to detect changes in the structure or expression level of genetic material in patients, including DNA, RNA and protein. Molecular diagnosis is the main method of predictive diagnosis. At present, Kayudi's molecular detection platform includes portable fluorescent quantitative PCR Mini8 Plus and "one-step" nucleic acid-free extraction reagents, which can be applied to the detection of many diseases and human genetics, including infectious disease screening and tumor related. Screening for pathogens and individualized medication guidelines. After the medical staff collects the blood or saliva sample for the user, the sample is placed in the PCR instrument, and the PCR instrument performs sample analysis and issues a test report at the computer terminal connected thereto. The complete test process takes approximately 20 minutes. And the "one-step" molecular detection platform is a fully enclosed system, which can avoid the artificial pollution caused by the experiment and improve the detection accuracy.
"Some cancers have a lot to do with viral infections. For example, cervical cancer has been confirmed to be caused by HPV infection, and infection with Helicobacter pylori HP increases the risk of gastric cancer. Therefore, early detection of related viruses can achieve cancer. Kaydi hopes that everyone can benefit from molecular diagnosis," said Li Xiang, founder and CEO of Kayudi.

The technology of nucleic acid detection has a high technical threshold, and Kayidi’s products detected the first imported Zika virus in Beijing.
Speaking of competition within the industry, Li Xiang said frankly that so far, no molecular testing company in the industry has a completely competitive relationship with Kayudi. Kayudi's technology is fast on-site molecular detection, which has certain advantages compared with the current state of the art in the nucleic acid detection market.
For the milestones in the company's development process, Li Xiang said that in early 2015, the Ebola virus in Africa was raging, and Kayudi's molecular detection platform was used to detect Ebola virus in Sierra Leone, contributing to the control of the epidemic. The World Health Organization (WHO) official website has included Kayudi's detection system in the list, and Kayudi has also become China's first supplier of nucleic acid testing equipment.
In 2016, Kayudi cooperated with the Chinese Center for Disease Control and Prevention to set up an on-site molecular testing platform at the Capital International Airport, Beijing West Railway Station, and Shanghai, Shenzhen, Zhejiang, Liaoning and other places for entry-exit inspection and quarantine agencies to screen Zika virus. Check the work. After the first detection of 4th and 5th cases of imported Zika infection in China, on May 14, 2016, Kayudi's detection platform detected the first imported Zika infection in Beijing at the Capital International Airport.

Kayudi has received two rounds of investment
Cayudi's founder and CEO Li Xiang is a bachelor of physics at Peking University and a master's in biophysics from the University of California. In 2006, Li Xiang established Jin Yin Xing Biotechnology and Gingko Biotechnology LLC in China and the United States respectively. COO Angela Zhang is a Ph.D. in biomedical science and has served as the VP of Safran Asia Fund. CTO Jesus Ching was the R&D Director of Cepheid, the first on-site molecular diagnostics company in the United States.
Kayudi won the A round of financing in 2014, and the funder is the Safran Asia Fund. In 2015, the company was led by Arctic Light Ventures, Safran Asia and Lenovo Star and investment in the B round of financing. The total amount of the two rounds of financing is about 20 million US dollars.
In the next step, Kayudi will continue to develop new products and gradually spread molecular testing products to primary medical institutions.
Source: Arterial Network
Cut-resistant Gloves and Sleeves
Cut-Resistant Gloves And Sleeves,Cut Proof Gloves,Anti Cut Gloves,Puncture Resistant Gloves
Jiangsu Hespax Security Co., Ltd , https://www.hespax.com