Nanmo Bio has constructed more than 15 mouse models for this study.
Highlight
Problem to be solved: How to label non-muscle cells and cardiomyocytes separately?
Solution:
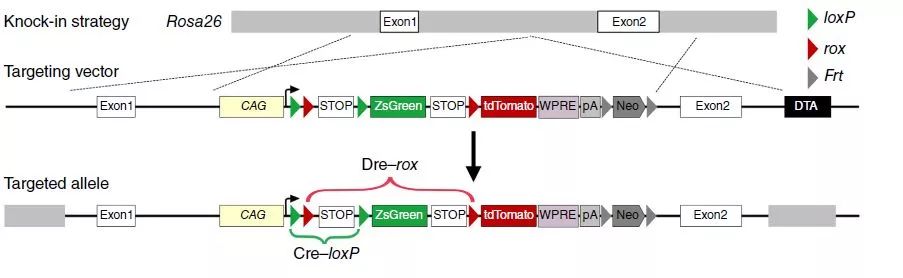
Using the DeaLT dual-recombinase-activated lineage tracing to combine the Dre-rox recombination system with the traditional Cre-loxP recombination system, the interlaced loxP and rox sites can be in Cre-loxP or After Dre-rox recombination, another set of recombinant systems is excised, and another set of recombinase recognition sites are excised in cells that may cause ectopic expression of the recombinase, thereby effectively preventing ectopic recombination and allowing cells to exclusively Labeled with ZsGreen or tdTomato fluorescence, increasing the accuracy of the lineage tracer results.
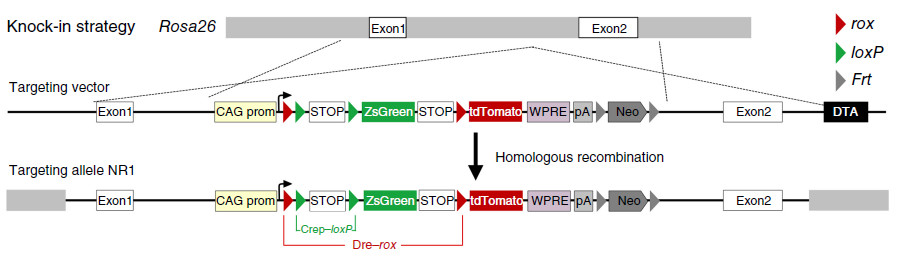
According to the order of position of loxP and rox, it is divided into IR and NR two kinds of reporter gene systems.
Option 1: IR
Cardiomyocytes were first labeled with tdTomato red fluorescence using Dre of cardiomyocyte marker;
Further, inducible Cre is used to express Cre recombinase after drug induction, and all non-cardiomyocytes except cardiomyocytes are permanently labeled with ZsGreen green fluorescence;
If non-muscle cells are transformed into muscle cells, the neonatal cardiomyocytes transformed from non-muscle cells will be ZsGreen green fluorescently labeled. Therefore, it is only necessary to find whether there is a cardiomyocyte with green fluorescence during myocardial regeneration.
Option 2: NR
First, the Dre of cardinal cells and the widely expressed Cre divide the cells into two types, one is the muscle cells expressing tdTomato red fluorescence, and the other is non-muscle cells expressing ZsGreen green fluorescence;
In the case of non-myogenic cell-to-muscle cell fate transformation, during the process of new myopoiesis, the Dyer of cardiomyocyte marker will mediate the original green fluorescent-labeled cells labeled with tdTomato red fluorescence, so both green and red will be present. New muscle cells with positive markers produce a yellow fluorescent signal.
Research Background
The heart, one of the most important organs of vertebrates, is primarily responsible for powering blood flow. Cardiac infarction causes a large number of myocardial cells to die and heart function is affected. The existence of cardiac stem cells in adult hearts has been controversial. Previous studies have used traditional genetic lineage tracing techniques to consider the presence of cardiac stem cells in adult hearts, such as Kit+ cardiac stem cells, Bmi1+ cardiac stem cells, Scal1+ cardiac stem cells, Islet+ cardiac stem cells, etc. The molecular markers of these cardiac stem cells are themselves expressed in part of the cardiomyocytes, so the differentiation potential of these putative cardiac stem cells to cardiomyocytes is still questioned.
In the latest study, the researchers used dual homologous recombination techniques to simultaneously trace lineages of muscle and non-muscle cells, revealing the potential of non-muscle cells to differentiate into muscle cells.
New tracer technology for labeling non-muscle cells
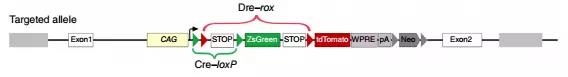
To clarify the presence of putative cardiac stem cell CSCs in non-muscle cells, the researchers designed a new system to label all non-muscle cell lineages. Using the DeaLT-IR1 reporter mouse (see figure below), the staggered loxP and rox sites can be resected after Cre-loxP or Dre-rox recombination, allowing the cell to be exclusively labeled with ZsGreen. Or tdTomato fluorescence.
How to use this system to label muscle cells and non-muscle cells separately? Tnnt2-Dre (Tnnt2 is a cardiomyocyte marker), R26-iCre (obtained by R26-rtTA and TRECre, can express Cre under the induction of doxycycline Dox) and the mice of the IR1 reporter mice are mated together. , obtained Tnnt2-Dre; R26-iCre; IR1 mouse: Tnnt2-Dre first labeled Dre-expressing myocytes; and after doxycycline (Dox) treatment, R26-iCre labeled all cells except muscle cells (Fig. 1A) , B). In the double homologous recombination system, muscle cells were labeled with tdTomato and non-muscle cells were labeled with ZsGreen.
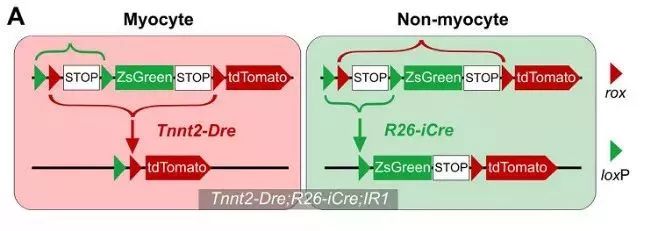
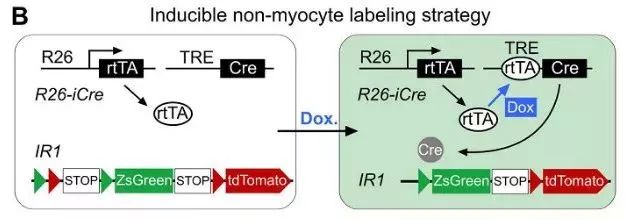
Figure 1. AB
First, studies in Africa transformed embryonic heart muscle cells into muscle cells with this system. In the early stage of embryonic heart development, Isl1+ cardiomyocytes can contribute to the cardiomyocytes of the second heart region. For example, the outflow tract of the heart, the cardiomyocytes of the right ventricle, and the very small amount of cardiomyocytes in the left ventricle and atrium are derived from the Is1+ cardiac muscle. Stem cells (Fig. 1C). Isl1-CreER; R26-tdTomato mice were given tamoxifen induction on day E8.0, and the right ventricle was marked with red fluorescence at E13.5 days (Fig. 1D).
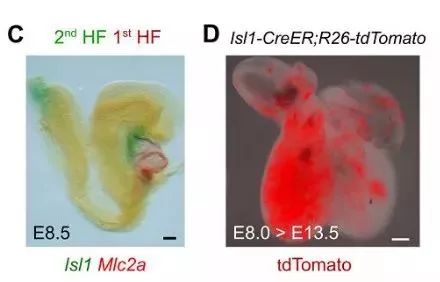
Figure 1. CD
Tnnt2-Dre; R26-iCre; IR1 mice were given Dox-induced marker non-myocytes on day E8.0 and were found to be labeled with ZsGreen green fluorescence in other tissues at E8.5 days, with red fluorescent markers on the myocardium (Fig. 1E). . Next, heart tissue was collected for E13.5 days, and immunostaining of dTomato, ZsGreen, and TNNI3 (myocyte marker) was found to show that most of the myocytes in the right ventricle were labeled with ZsGreen green fluorescence at E13.5 days, indicating in the embryonic heart. In early development, non-cardiomyocytes have the ability to differentiate into cardiomyocytes, which can contribute to the formation of cardiomyocytes in the second heart region (Fig. 1F), demonstrating that the new tracer system can effectively detect developing cardiac non-muscle cells. Transformation to cardiomyocytes.
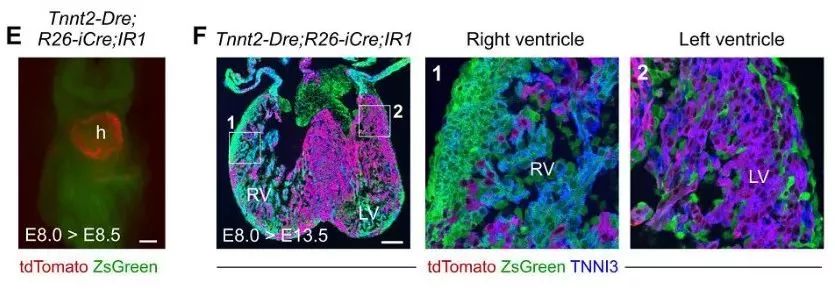
Figure 1. EF
Revealing 4 strategies for non-muscle cell transdifferentiation
Strategy 1 Tnnt2-Dre; R26-iCre; IR1 non-muscle cells do not contribute to myocytes in adult hearts
Tnnt2-Dre; R26-iCre; IR1 mice were used to simultaneously track cardiomyocytes and non-cardiomyocytes in adult hearts. Immunostaining of tdTomato, ZsGreen and TNNI3 showed that tdTomato+ cells were TNNI3+ cardiomyocytes, whereas ZsGreen+ cells were not TNNI3+ cardiomyocytes (Fig. 1G). Isolation of Tnnt2-Dre; R26-iCre; IR1 cardiomyocytes did not detect ZsGreen+ cardiomyocytes in vitro (Fig. 1H). Thus, the Tnnt2-Dre; R26-iCre; IR1 system labeled myocytes and non-muscle cells with two different fluorescent colors (Fig. 1I).
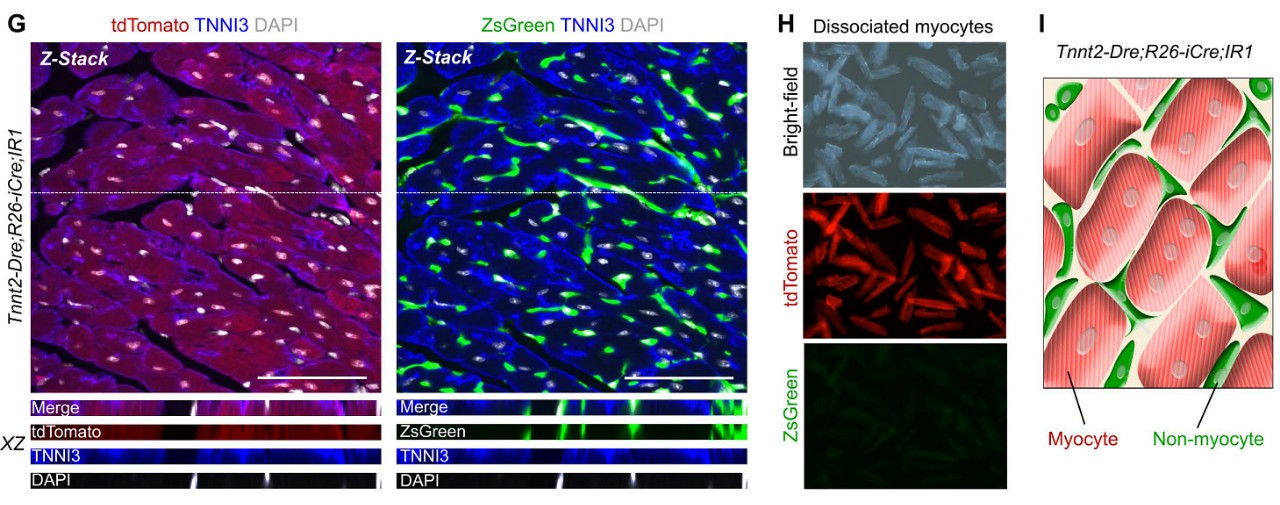
Figure 1. GI
Does non-muscle cells differentiate into myocytes in adult hearts? If so, the new cardiomyocytes derived from non-muscle cells will be ZsGreen+. The results of the experiment were: after Dox induction, Tnnt2-Dre; R26-iCre; IR1 mice were ligated to the left anterior descending coronary artery to induce MI modeling (Fig. 2A). After myocardial infarction, a large number of myocardial cells died and cardiac function was impaired. ZsGreen, tdTomato and TNNI3 immunostaining were performed on heart sections 2-4 weeks after injury, and no ZsGreen+TNNI3+ cardiomyocytes were found (Fig. 2B). ZsGreen+tdTomato-cardiomyocytes were not detected in more than 600 sections of 5 myocardial infarction tissues. Flow cytometric analysis (Fig. 2D) and fluorescence microscopy (Fig. 2E) also confirmed that ZsGreen+ cardiomyocytes were not observed in the hearts of Tnnt2-Dre; R26-iCre; IR1 mice after MI modeling. The RT-PCR results also showed only tdTomato expression and no ZsGreen mRNA expression (n=5, Figure 2F). These data indicate that non-cardiomyocytes do not contribute to muscle cells after cardiac damage in adult hearts.
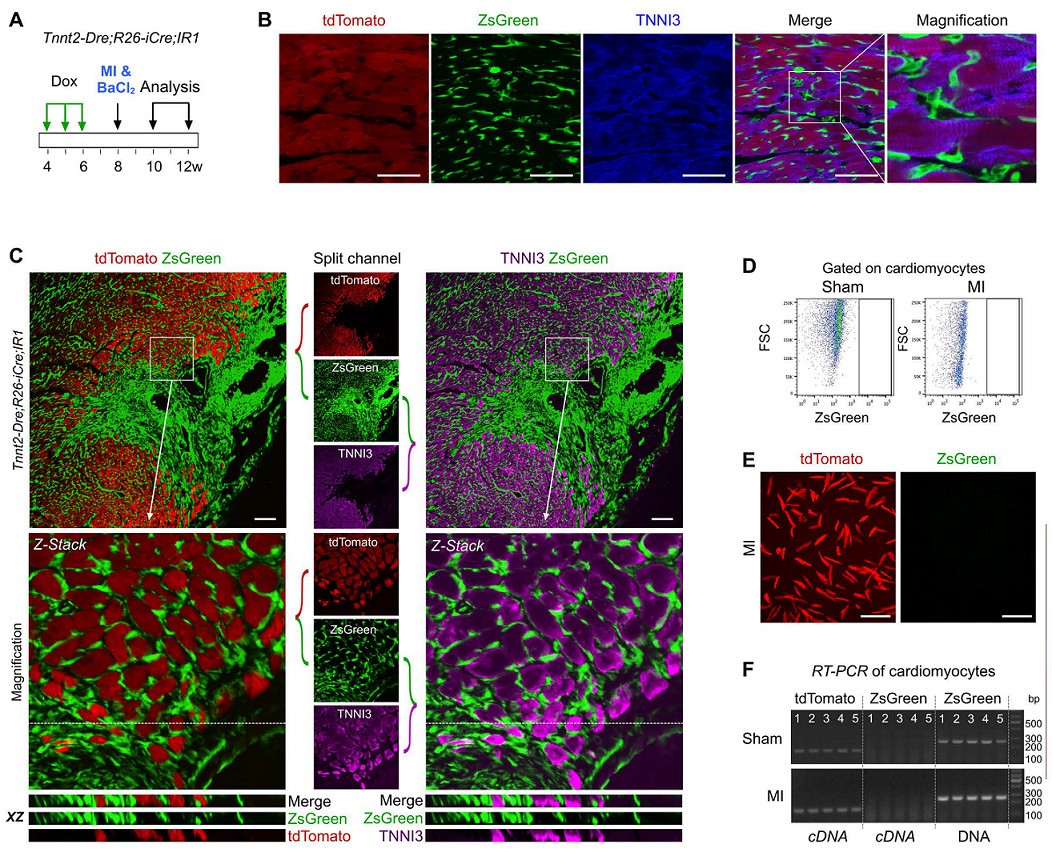
Figure 2. AF
In parallel, the cell fate of non-muscle cells to muscle cells in skeletal muscle after skeletal muscle injury was converted as a positive control. During the steady state maintenance of skeletal muscle, myofibroblasts are fused with non-muscle cells, and myofibroblasts are in a multinuclear state. Therefore, in normal skeletal muscle (sham operation group), tdTomato+ZsGreen+ muscle cells can be detected (Fig. 2G). When BaCl2 injury was applied to the tibialis anterior muscle (TA muscle) of Tnnt2-Dre;R26-iCre;IR1 mice, muscle fiber cells in skeletal muscle died in large numbers, and weak tdTomato fluorescence signal and strong ZsGreen expression were detected after TA muscle injury. It was shown that ZsGreen+ cells gradually replaced tdTomato+ cells after BaCl2 modeling (Fig. 2G). ZsGreen+tdTomato-muscle cells (arrow, Figure 2G) were detected in damaged TA muscle compared to the sham-operated group, indicating that non-muscle cells re-formed muscle cells.
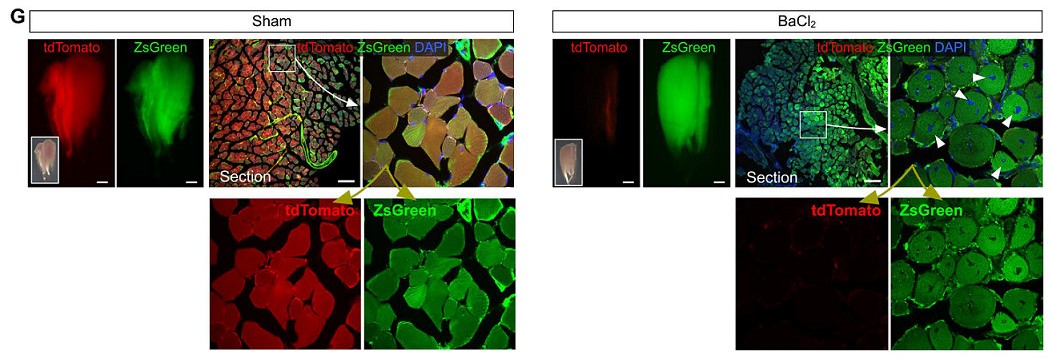
Figure 2. G
Combining the above results, the dual homologous recombination lineage tracing technique of Tnnt2-Dre;R26-iCre;IR1 (strategy 1) revealed the transformation of non-muscle cells into myocytes in embryonic heart and adult skeletal muscle, but in adult heart Neutral myocytes do not contribute to myocytes.
Strategy 2 Tnnt2-Cre; R26-DreER; IR3 Reverse Cre/Dre System Labels Two Cells in Non-Myocyte to Myocyte Transformation Studies
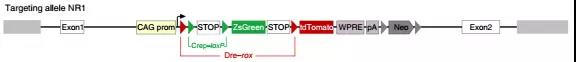
A second double homologous recombination lineage tracing system was designed to validate the transformation of non-myocytes into myocytes. The system consists of Tnnt2-Cre (Cre expressed in myocytes), tamoxifen-inducible R26-DreER and IR3 reporter mice. The non-muscle cells were labeled with tdTomato red fluorescence exclusively by IR3 reporter mice (see figure below), and the muscle cells were specifically labeled with ZsGreen green fluorescence.

Tnnt2-Cre; R26-DreER; IR3 mice can simultaneously label muscle cells (ZsGreen+) and non-muscle cells (tdTomato+) (Fig. 3A).
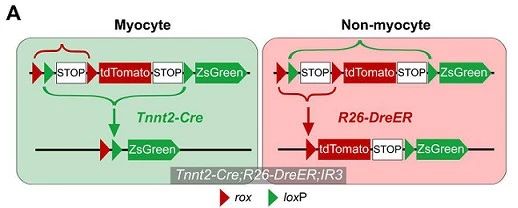
Figure 3. A
ZsGreen was primarily labeled with the original cardiac tube in the embryo's E8.0 day heart (Fig. 3B). Tamoxifen was induced on day E8.0, and tdTomato+ non-muscle cells were found to have a significant contribution to muscle cells in the right ventricle at E13.5 days (Fig. 3C). This result was also confirmed by immunostaining of heart sections (Fig. 3D-F).
Similarly, in adult hearts and skeletal muscle, the transformation of fate of non-cardiomyocytes was observed by MI and BaCl2 modeling (Fig. 3G). Flow cytometric analysis (Fig. 3H) and fluorescence microscopy (Fig. 3I) of isolated cardiomyocytes also showed no tdTomato+ cardiomyocytes, respectively. Morphologically, these tdTomato+ cells were also significantly different from ZsGreen+ cardiomyocytes (Fig. 3J).
Immunostaining of tdTomato and TNNI3 showed that these tdTomato+ cells were not transformed into TNNI3+ cardiomyocytes in the infarct, border and distal regions of the MI heart (Fig. 3K). TdTomato+TNNI3+ cardiomyocytes were not detected in more than 600 tissue sections from 5 Tnnt2-Cre; R26-DreER; IR3 mouse hearts. RT-PCR results also indicated that isolated cardiomyocytes expressed only ZsGreen and did not express tdTomato (Fig. 3L).
In contrast, in positive control experiments, tdTomato+ZsGreen-muscle cells (arrows, Figure 3M) were easily detected after tamoxifen induction in the TA muscle injury model, whereas the sham group did not.
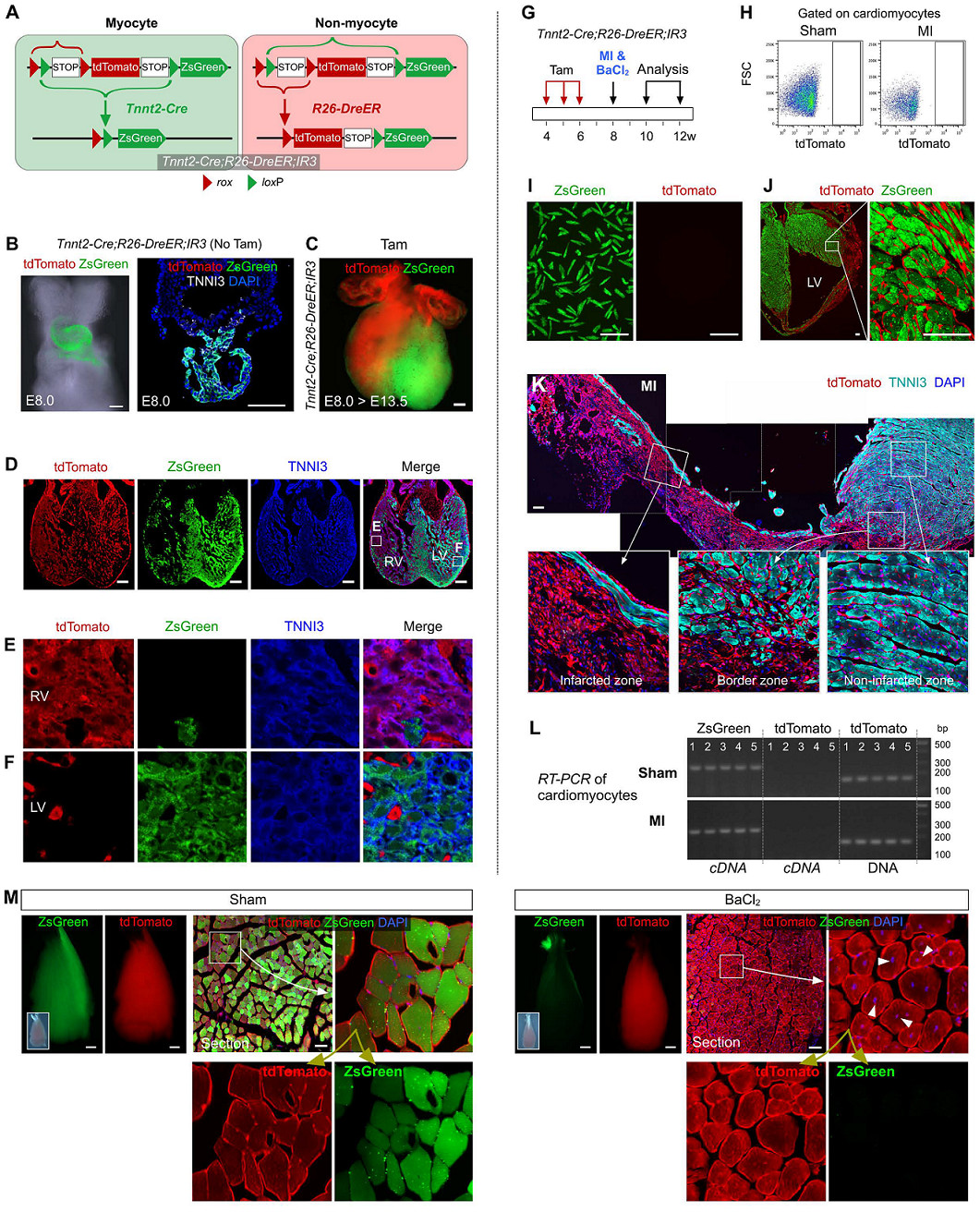
image 3.
Combining the above results, the second strategy uses Tnnt2-Cre; R26-DreER; IR3 also shows results consistent with strategy 1: non-cardiomyocytes are converted to myocytes in embryonic heart and adult skeletal muscle, but in adult hearts Myocytes did not contribute.
Strategy 3 Tnni3-Dre; R26-iCre; IR1
To further verify the above findings, another muscle cell marker, TNNI3, was used to drive the Dre recombinase. Tnni3-Dre effectively and specifically labels muscle cells in adult hearts, but does not label non-muscle cells. Muscle cells and non-muscle cells were labeled with Tnni3-Dre; R26-iCre; IR1 mice, respectively (Fig. 4A).
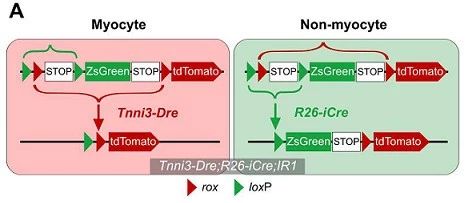
Figure 4. A
Immunostaining by ZsGreen, tdTomato, and different cardiac cell line markers showed that myocytes express tdTomato, non-muscle cells (eg, endothelial cells, fibroblasts, etc.) express ZsGreen (supplemental material). No ZsGreen+ cell tissue was observed in Tnni3-Dre; R26-iCre; IR1 mice without Dox induction (Fig. 4B).
Two weeks after Dox administration, MI or BaCl2 injection (Fig. 4C) was performed, and it was found that in the sham-operated heart, tdTomato+ cardiac cells were also labeled with ZsGreen green fluorescence after Dox induction (Fig. 4D). Immunostaining of tdTomato, ZsGreen and TNNI3 showed that all cardiomyocytes were tdTomato+ZsGreen- (Fig. 4E). After MI modeling, the number of tdTomato+ myocytes in the infarcted heart tissue was significantly reduced and replaced by ZsGreen+ non-muscle cells (Fig. 4F).
The myocardial cells after separation of MI were observed in vitro as tdTomato+ZsGreen- cells (Fig. 4G). Immunostaining of tdTomato, ZsGreen and TNNI3 showed that ZsGreen+ cells did not turn to cardiomyocyte fate after injury (Fig. 4H, I). No ZsGreen+tdTomato-muscle cells were detected in the MI heart by split flow cytometry and RT-PCR (Fig. 4J, K).
In contrast, in positive control experiments, ZsGreen+tdTomato-muscle cells (arrow, Figure 4L) were detected in injured TA muscle, indicating that non-myocyte neoplasms are myocytes.
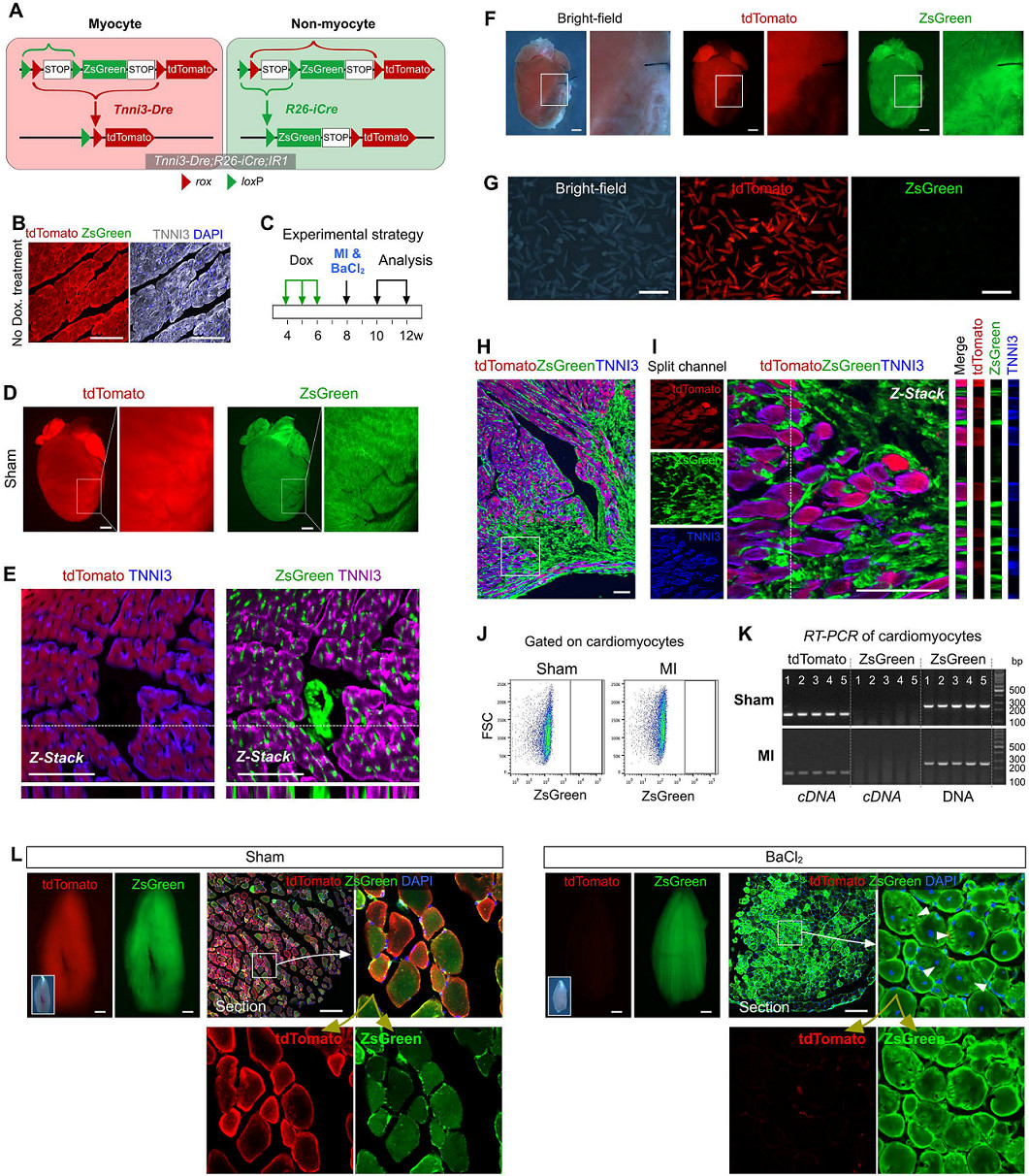
Figure 4.
Therefore, the third model based on Tnni3-Dre; R26-iCre; IR1 also revealed that non-muscle cells do not transform into myocytes in adult hearts.
Strategy 4 Tnnt2-Dre; Actb-Cre; NR1 studies the transformation of non-muscle cells into myocytes through the NR1 system
Although inducible Cre or Dre driven by a broad promoter can effectively label most non-muscle cells, in practice the labeling efficiency does not reach 100%. It is still possible that a small number of unlabeled non-muscle cells produce new muscle cells in the adult heart after injury, although the likelihood is not significant because the labeling process during lineage tracing is completely random. To achieve 100% non-muscle cell labeling efficiency, a fourth strategy was designed using NR reporter mice (see figure below).
In this system, ZsGreen labels all cells in mice except for newly formed muscle cells labeled with tdTomato. All cells in NR1 mice after Actb-Cre-loxP recombination were first labeled as ZsGreen green fluorescence, and only new myocytes were labeled with tdTomato red fluorescence upon formation due to recombination of the second Tnnt2-Dre-rox (Fig. 5A). Green fluorescent protein has a half-life of 12-24 hours in mammalian cells, so although Dre-rox recombination in myocytes leads to tdTomato expression, ZsGreen protein still has a stable labeling time before degradation. Therefore, double positive cells can be detected during the transformation of non-muscle cells into muscle cells.
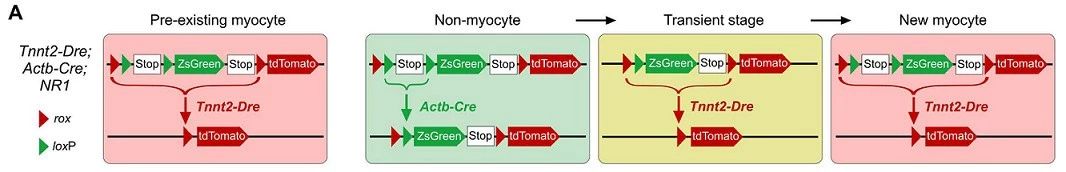
Figure 5. A
The system was first tested by analyzing the embryonic heart of the previous experiment (Fig. 5B). It is easy to detect tdTomato+ZsGreen+ myocytes in the heart outflow tract developed in E8.5 days (arrow, Fig. 5C), which is consistent with previous studies, indicating that the second heart region progenitor cells differentiate into cardiomyocytes at this stage.
Mouse heart samples were then collected every 12 hours during the 28 days of MI modeling for analysis (Fig. 5B). For ZsGreen, tdTomato and TNNI3 immunostaining, the results showed no ZsGreen+tdTomato+ muscle cells in the infarcted myocardium (Fig. 5D). At all 56 time points, Tnnt2-Dre; Actb-Cre; NR1 mouse heart samples, no ZsGreen+tdTomato+ muscle cells were detected in more than 6000 heart sections. Similarly, isolation of cardiomyocytes for flow cytometry did not reveal muscle cells in ZsGreen+ cells (Fig. 5E).
In contrast, ZsGreen+tdTomato+ myocytes were easily detected in the injured TA muscle in the positive control experiment (Fig. 5F). Non-muscle cells were 100% labeled with ZsGreen (supplemental material) in Tnnt2-Dre; Actb-Cre; NR1 mouse heart sections.
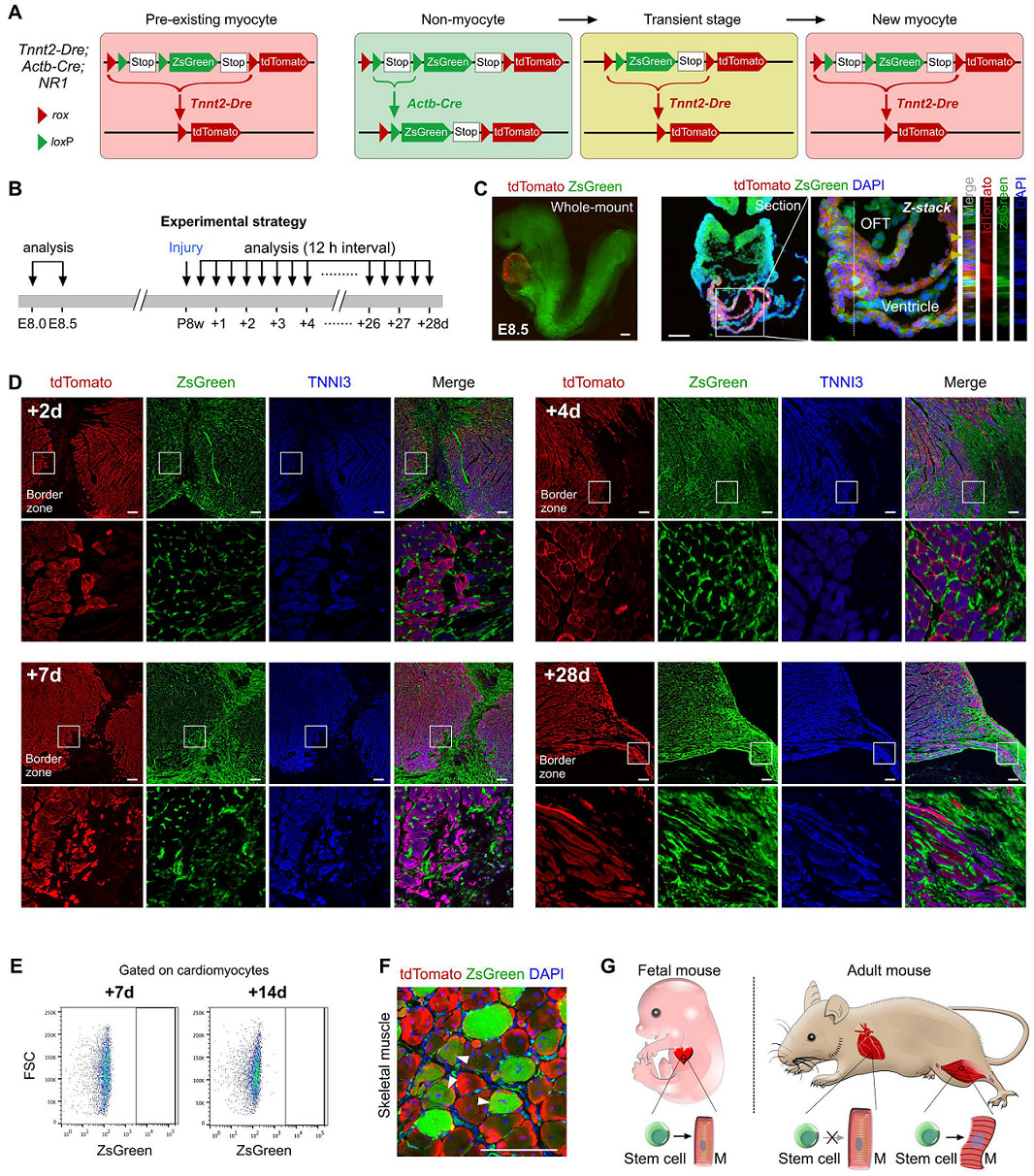
Figure 5.
In summary, the results of the fourth lineage tracing strategy based on Tnnt2-Dre; Actb-Cre; NR1 showed that the transformation of non-muscle cells into myocytes appeared only in the embryonic phase and not in the adult heart.
Contribution of different cardiac cell lineages to muscle cells
In addition to cardiomyocytes in adult hearts, there are a variety of non-muscle cell lineages with known molecular markers. For these different cell populations, different Marker promoters were used to drive the recombinase building tool mouse to track the cell fate of non-muscle cells, such as:
Inner cell: Npr3-CreER;
Mesenchymal stem cells or fibroblasts: Sox9-CreER, Vim-CreER, Postn-CreER;
Endothelial cells: Cdh5-CreER;
Pericytes or smooth muscle cells: Myh11-CreER, Pdgfrb-CreER, NG2-CreER;
Epicardial cells: Wt1-CreER;
As a control, aMHC-MerCreMer was used to trace cardiomyocytes.
Tamoxifen induction was performed on these 10 tool mice, MI modeling or sham operation was performed two weeks later, and cardiac tissue samples were collected 4 weeks after cardiac injury (see Figure B below). TdTomato and myocyte marker TNNI3 staining on heart sections of these 10 mouse strains showed no contribution from myopic cell lineage to these different cell lineages except for existing cardiomyocytes after sham surgery or MI ( Figure C, D) below. These results indicate that all of these non-muscle cells are not involved in the transformation to new myocytes, and aMHC-MerCreMer trace data show that all cardiomyocytes in the damaged myocardium are derived from existing cardiomyocytes (Figure C, D below) ).
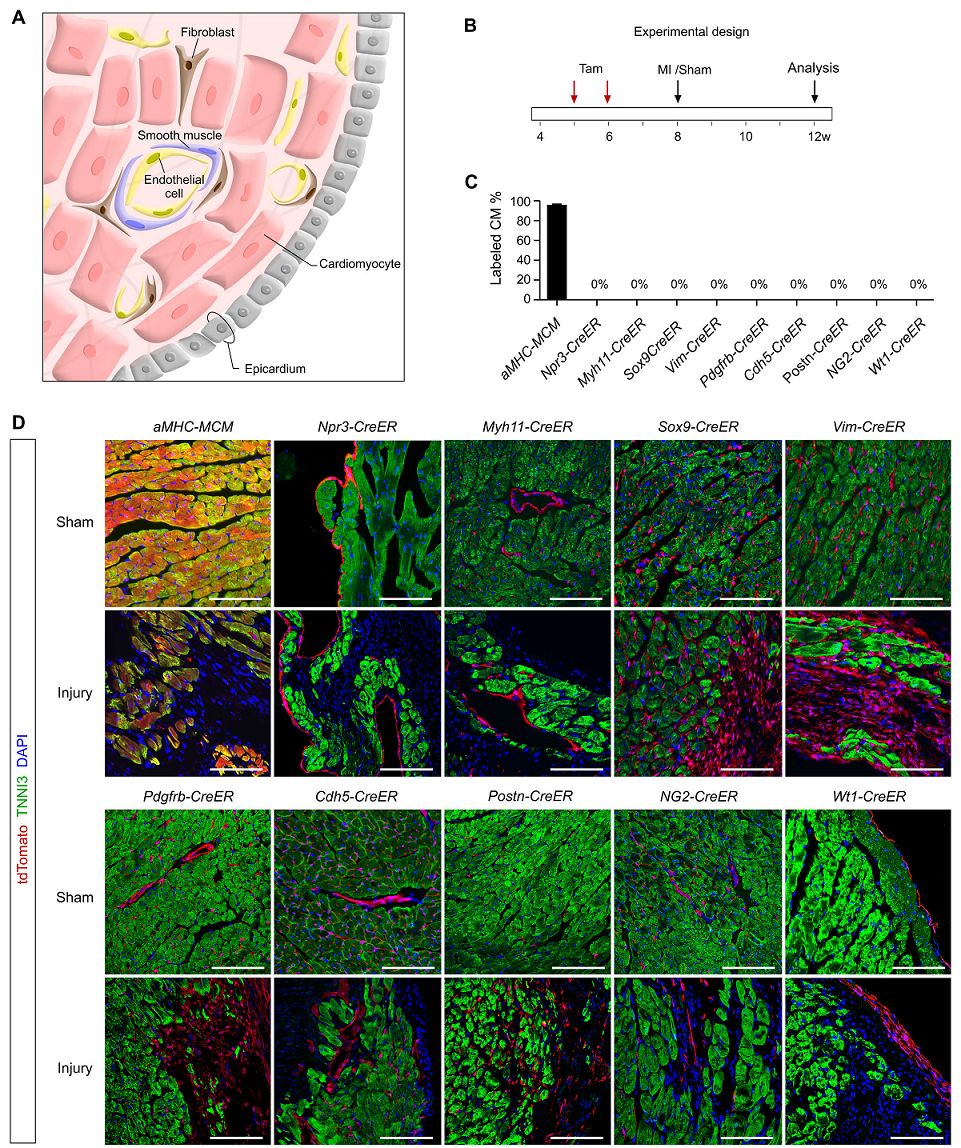
Figure 6. AD
Analysis conclusion
Therefore, combining all the experimental results, muscle cells produce myocytes in myocardial regeneration, and there is no transformation of non-muscle cells into muscle cells.
What is the clinical significance of this study using the newly established dual homologous recombination technology system?
It is shown that the putative Sca1+, Bmi1+, Isl1+, Abcg2+ or other stem cell population does not have myogenic potential for cardiac regeneration in the adult stage.
Cardiomyocytes are the main source of cells for cardiac regeneration.
This new genetic system provides an unprecedented strategy to study the contribution of putative adult stem cells to tissue repair and multi-organ system regeneration.
' + $title + '
'); var printArea = $content.html(); window.document.body.innerHTML = printArea; window.print(); window.document.body.innerHTML = bodyHtml; return true; }); }); ;( Function($){ $.fn.totop=function(opt){ var scrolling=false; return this.each(function(){ var $this=$(this); $(window).scroll(function(){ If(!scrolling){ var sd=$(window).scrollTop(); if(sd>200){ $this.fadeIn(); }else{ $this.fadeOut(); } } }); $this. Click(function(){ scrolling=true; $('html, body').animate({ scrollTop : 0 }, 500, function(){ scrolling=false; $this.fadeOut(); }); }); }); }; })(jQuery); $("#backtotop").totop(); }); (function(a,h,c,b,f,g){a["UdeskApiObject"]=f ;a[f]=a[f]||function(){(a[f].d=a[f].d||[]).push(arguments)};g=h.createElement(c) ;g.async=1;g.charset="utf-8";g.src=b;c=h.getElementsByTagName(c)[0];c.parentNode.insertBefore(g,c)})(window, Document,"script","//assets-cli.udesk.cn/im_client/js/udeskApi.js","ud"); ud({ "code": "2615360h", "link": "//modelorganisms .udesk.cn/im_client/?web_plugin_id=45320", "selec Tor": "#udesk" }); var _hmt = _hmt || []; (function() { var hm = document.createElement("script"); hm.src = "https://hm.baidu.com /hm.js?14e3c5484a7e92ce4b5348385460227d"; var s = document.getElementsByTagName("script")[0]; s.parentNode.insertBefore(hm, s); })(); $(document).ready(function () { $('#guestbook-submit').click(function () { $('#guestbook-form').ajaxSubmit({ url: "/plugin/sy_guestbook/index/ Addmsg.html", type: "post", success: function (data) { if (data.code == 0) { layer.msg(data.msg); } else { $('#MessageSent').removeClass( "d-none"); layer.msg(data.msg, function () { setTimeout(function () { parent.location.reload(); }, 300); }); } } }); return false; } ); });Cervical Support Brace,Cervical Vertebra Tractor,Best Cervical Traction Device,Cervical Neck Traction Device
Henan Anbang Medical Supplies Co., Ltd. , https://www.anbangmedical.com