DPP-4 is an enzyme in the body, that is, an enzyme. Its main role is to break down the body's proteins. One of the proteins degraded by DPP-4 is called GLP-1. It is a hormone secreted by intestinal cells. GLP-1 can be stimulated by insulin, inhibiting glucagon, inhibiting gastric emptying and regenerating islet cells. To lower blood sugar. At present, DPP-4 inhibitors that cause inactivation of DPP-4 so as not to decompose GLP-1 have become one of the main directions for the treatment of diabetes.
In the United States, Genovex (general name: sitagliptin phosphate) launched in the United States in 2006 was the first product to introduce a DPP-4 inhibitor, and Novartis's Jiaweile (vildagliptin) was delayed due to side effects. Since then, more products of the same type, such as Anzale (sazagliptin) and tradjenta (linagliptin), have also entered the market one after another, bringing more market momentum to the therapeutic field.
In the following five years, DPP-4 inhibitors accounted for 33% of the world's non-insulin diabetes drug use, and in developed countries, the market share of DPP-4 inhibitors in diabetes drug use has grown to 58%.
A related report from IMS pointed out that Merck has delayed the launch of its compound preparations, Sinodag (Sitagliptin Phosphate and Metformin Hydrochloride), until the launch of its single compound product, Jenova, a year later. This means that Tenoda will be the second compound product on the market in this sector. In fact, Genoda's IPO in the UK has been lagging behind Novartis's Eucles (Widgliptin and Metformin) for a full two years.
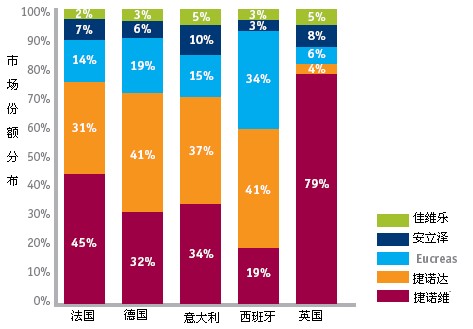
As for Eucuras, it does not cover the five countries in the EU as well as Genoda, mainly because Tenoda has a relatively stable patient market in the region (as shown below). However, in terms of Novartis family products, Eucreas's sales performance in most European markets is much better than that of Jiaweile (Vidextin), especially in the 2011 DPP-4 inhibitor market in Spain. Eucreas holds 34% of the market, while Jiaweile has only 3%.
Figure 1: Market Share of DPP-4 Inhibitor EU 5 Countries
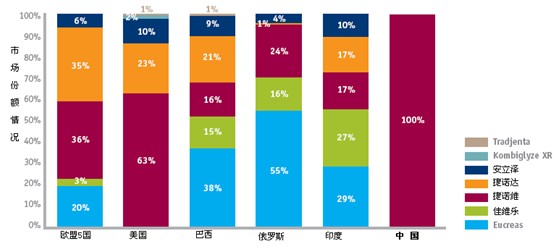
The first-line products in this field are also active in the major emerging markets. In Russia, Brazil and India, Novartis’ products accounted for more than half of the market for DPP-4 inhibitors, with Eucias being the largest contributor. In India, Jiaweile’s market share is 10% higher than that of Jinouwei. Before 2011, only China’s Zenitron, which is Merced, is listed.
Figure 2: Sales Share of Emerging Markets for DPP-4 Inhibitors
The products currently approved for listing in China are shown in the table below. Since Zenovo was listed in 2009 and Other products were only approved in 2011 and later, there is currently only sales data for the company.
Table 1: Approval of DPP-4 inhibitors in China
Product Name (English) Common Name Manufacturer SFDA Approved Approval Time JANUVIA Sitagliptin Phosphate Tablet MSD 2009 Onglyza Saxagliptin Tablets Bristol-Myers Squibb 2011 Galvus Novartis tablets vildagliptin 2011 Jienuo Da (JANUMET) sitagliptin metformin Merck 2012
In the United States, Genovex (general name: sitagliptin phosphate) launched in the United States in 2006 was the first product to introduce a DPP-4 inhibitor, and Novartis's Jiaweile (vildagliptin) was delayed due to side effects. Since then, more products of the same type, such as Anzale (sazagliptin) and tradjenta (linagliptin), have also entered the market one after another, bringing more market momentum to the therapeutic field.
In the following five years, DPP-4 inhibitors accounted for 33% of the world's non-insulin diabetes drug use, and in developed countries, the market share of DPP-4 inhibitors in diabetes drug use has grown to 58%.
A related report from IMS pointed out that Merck has delayed the launch of its compound preparations, Sinodag (Sitagliptin Phosphate and Metformin Hydrochloride), until the launch of its single compound product, Jenova, a year later. This means that Tenoda will be the second compound product on the market in this sector. In fact, Genoda's IPO in the UK has been lagging behind Novartis's Eucles (Widgliptin and Metformin) for a full two years.
As for Eucuras, it does not cover the five countries in the EU as well as Genoda, mainly because Tenoda has a relatively stable patient market in the region (as shown below). However, in terms of Novartis family products, Eucreas's sales performance in most European markets is much better than that of Jiaweile (Vidextin), especially in the 2011 DPP-4 inhibitor market in Spain. Eucreas holds 34% of the market, while Jiaweile has only 3%.
Figure 1: Market Share of DPP-4 Inhibitor EU 5 Countries

The first-line products in this field are also active in the major emerging markets. In Russia, Brazil and India, Novartis’ products accounted for more than half of the market for DPP-4 inhibitors, with Eucias being the largest contributor. In India, Jiaweile’s market share is 10% higher than that of Jinouwei. Before 2011, only China’s Zenitron, which is Merced, is listed.
Figure 2: Sales Share of Emerging Markets for DPP-4 Inhibitors

The products currently approved for listing in China are shown in the table below. Since Zenovo was listed in 2009 and Other products were only approved in 2011 and later, there is currently only sales data for the company.
Table 1: Approval of DPP-4 inhibitors in China
Product Name (English) Common Name Manufacturer SFDA Approved Approval Time JANUVIA Sitagliptin Phosphate Tablet MSD 2009 Onglyza Saxagliptin Tablets Bristol-Myers Squibb 2011 Galvus Novartis tablets vildagliptin 2011 Jienuo Da (JANUMET) sitagliptin metformin Merck 2012
elastic band machine 1,High Quality elastic band machine 1,elastic band machine 1 Details, CN
Dongguan Huitong Automatic Machinery Technology Co., Ltd , https://www.breezesolution.com