Recent research progress in the field of central nervous system diseases (04.11)
April 11, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. Sunovion : Parkinson's disease oral new drug submitted for marketing application, fast track qualification
Recently, Sunovion announced that it has submitted a new drug application (NDA) for apomorphine sublingual membrane (APL-130277) to the US FDA for the treatment of motion fluctuations (OFF events) in patients with Parkinson's disease (PD). The FDA also granted its fast track qualifications.

Parkinson's disease (PD) is a chronic progressive neurodegenerative disease with tremors, stiffness, and impaired movements at rest, and non-motor symptoms including cognitive and mood disorders. PD is the second most common neurodegenerative disease after Alzheimer's disease, affecting more than 1 million people in the United States and 4-6 million people worldwide. Its incidence increases with the aging of the population. The OFF event experienced by PD patients is the recurrence of symptoms (exercise and non-exercise) in the case of medication. It usually occurs in the morning and occurs regularly during the day. The OFF event manifests as tremor, stiffness, or bradykinesia, which can severely disrupt the patient's ability to perform daily activities, placing a huge burden on the patient and family. According to statistics, 40%-60% of PD patients will have an OFF event, and its frequency and severity may worsen during disease progression. These patients urgently need effective treatment to alleviate the disease and improve their quality of life.
Apomorphine sublingual membrane is a novel formulation of the dopamine agonist apomorphine and is currently being developed as a fast-acting sublingual membrane for on-demand management of PD-related OFF events. Apomorphine is currently the only subcutaneous injection approved in the United States for acute intermittent treatment of low-activity OFF events associated with advanced PD. Apomorphine sublingual membranes are designed to provide a potential treatment for PD-related OFF events, up to 5 times a day. It can quickly help patients switch from OFF to ON.

â–²Sunovion's R&D pipeline (Source: Sunovion Official Website)
The NDA submitted by Sunovion is based on the results of a key phase 3 study of CTH-300. This 12-week, randomized, double-blind, placebo-controlled, parallel-group, phase 3 study evaluated the efficacy and safety of apomorphine sublingual membrane in PD patients who responded to levodopa and experienced an OFF event. And tolerance. The primary endpoint of the study was the mean change from the pre-dose to 30-minute post-dose exercise for the dyskinesia social unified Parkinson's Disease Rating Scale (MDS-UPDRS) Part I during the 12th week of the maintenance treatment period. The key secondary endpoint for prospective designation was the proportion of patients who had a complete ON response within 30 minutes of the maintenance treatment period at the 12th week of the maintenance treatment period.
The results showed that the study reached the primary and secondary endpoints. Apomorphine sublingual membranes showed superior efficacy to placebo in the treatment of PD-related OFF events, and this effect continued until the last observation point of 90 minutes. In addition, the apomorphine sublingual membrane is also well tolerated.
"OFF events characterized by symptoms such as tremors, stiffness, or bradykinesia can disrupt a patient's ability to perform daily activities, burdening patients, families, and caregivers," Sunovion Executive Vice President, Chief Medical Officer and Sumitomo Dainippon Pharma Group Dr. Antony Loebel, Global Head of Clinical Development, said: "We are pleased to be able to submit the NDA of apomorphine sublingual membrane for the treatment of OFF events and look forward to working with the FDA during the review."
2. Allergan : Phase 3 clinical trial of new antipsychotic drugs
Recently, Allergan and Gedeon Richter announced that the cariprazine developed by the two parties reached the primary end point in the clinical phase III trial for the treatment of depression associated with type 2 bipolar I disorder. So far, cariprazine has shown significant efficacy in three key clinical trials for patients with bipolar disorder.

Bipolar disorder is a disease that can cause mood, vitality, and severe fluctuations in the ability to perform daily tasks. Patients may exhibit extreme extreme arrogance and depression. Patients with type 1 bipolar disorder will have a mad episode that lasts for at least 7 days, and a depressive episode usually lasts for at least 2 weeks. Usually patients need to be treated with psychotherapy and other drugs to control their condition. Few drugs can control the patient's manic and depressive symptoms at the same time.
Cariprazine, jointly developed by Allergan and Gedeon Richter, is an oral antipsychotic that has been approved by the FDA for the treatment of schizophrenia and can be used for acute treatment of adult type 1 bipolar disorder patients. Manic episodes of symptoms.
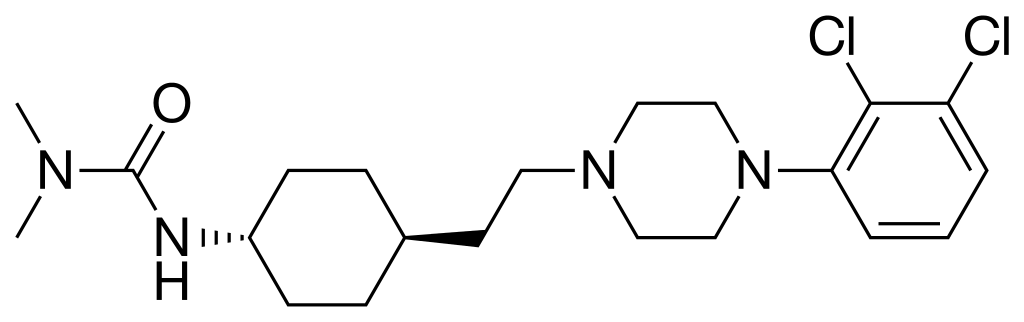
â–²Cariprazine molecular formula (Source: By Ed (Edgar181) (PubChem) [Public domain or Public domain], via Wikimedia Commons)
In this placebo-controlled randomized clinical phase 3 trial called RGH-MD-53, 493 patients received different doses of cariprazine or placebo. The test results showed that cariprazine reached the primary and critical secondary endpoints of the trial at a dose of 1.5 mg/day. The Montgomery-Asberg Depression Rating Scale (MADRS) was used to significantly improve the patient's depressive symptoms after 6 weeks of treatment with cariprazine (p=0.0417). The patient's assessment was also significantly improved with the Clinical Global Impression Scale-Severity (CGI-S) (p=0.0417).
Cariprazine showed good tolerance in clinical trials. 51% of patients treated with cariprazine had side effects, and 46% of patients who received placebo had side effects. Most of the side effects were mild or moderate, resulting in 5% of patients in the cariprazine-treated group discontinuing treatment, and 3% of patients in the placebo group discontinued treatment because of side effects.
“Treatment of bipolar disorder is a difficult task because there are few treatments that can control a patient's symptoms. Moreover, fewer products can comprehensively treat all symptoms from depression to mania,†Harvard Medical School (Harvard Medical School) Dr. Gary Sachs, professor of clinical psychiatry at University Medical School, said: "The data from this trial is an exciting news for patients and the wider psychiatric community that it proves that cariprazine has the potential to treat all the symptoms of the disease."

â–² Dr. David Nicholson, Chief Development Officer of Allergan Corporation (Source: Allergan Official Website)
To date, the efficacy of cariprazine in the treatment of depression associated with type 1 bipolar disorder has been demonstrated in a clinical phase 2 and two clinical phase 3 trials. Dr. David Nicholson, chief research officer at Allergan, said the company will include data from these clinical trials in a new drug application (NDA) filed with the FDA and is expected to be submitted in the second half of 2018.
3. Denali : New antibodies can shrink amyloid plaques
Plaques caused by the accumulation of β-amyloid (Aβ) in the brain are typical of AD patients, which is why many drugs and therapies are intended to target Aβ, but so far, they have not been in clinical phase 3 trials. A successful therapy.
â–²Image source: 123RF
Recently, researchers at the Washington University School of Medicine have found an effective way to clear amyloid plaques: targeting apolipoprotein E (APOE) genes with antibodies, known. Variants of the gene increase the risk of AD. Although the main component of plaque formation in AD is Aβ, there are also a small number of proteins encoded by the APOE gene. The researchers found that targeting the APOE gene cleared large numbers of plaques in the AD mouse model. Related papers were published in the Journal of Clinical Investigation.
APOE is a gene that provides prognosis in patients with AD. In the brain, APOE helps transport cholesterol to neurons to support their normal function. The APOE gene has three alleles called ε2, ε3 and ε4. Previous studies have found that the ε4 allele is associated with high risk and early onset of AD. It is estimated that up to 65% of AD patients in the US carry an APOE ε4 allele, while 10-15% of AD patients (about 560,000 in the US) have a genotype of APOE ε4 homozygote. APOE ε4 homozygous carriers exhibited faster rates of cognitive decline at multiple stages of the disease, and they also exhibited higher and faster amyloid accumulation.

In this new study, the researchers tested several antibodies targeting the APOE protein using mice with the human APOE gene. They developed these antibodies along with Denali Therapeutics, based in South San Francisco, California. Within six weeks, the researchers injected mice weekly with antibodies or placebo and continued to measure their brain plaques. The results showed that an antibody called HAE-4 reduced the plaque load in the brain of experimental mice by half. More importantly, this antibody only affects APOE protein levels in the brain without affecting APOE in the bloodstream. This is important because normal APOE proteins help the body manage fat and cholesterol, and eliminating it outside the brain can create unwanted off-target effects.
"It turns out that APOE in brain plaques has a different structure than APOE in the blood," said Dr. David Holtzman, director of the Department of Neurology at the University of Washington. "HAE-4 antibodies specifically recognize APOE proteins attached to plaques in the brain. form."
Previous anti-Aβ antibody development results are not satisfactory, and they may cause brain inflammation. However, anti-APOE antibody results may differ: According to Dr. Holtzman, anti-APOE antibodies may have fewer side effects because the APOE protein content in amyloid plaques is very low and the drugs do not bind too tightly. “This means we may find lower levels of immune activation and will not see unwelcome side effects,†commented Dr. Holtzman. The next step for the research team is to test similar antibodies and design further tests to determine if they are safe for humans.
Note: The title map source 123RF
Reference materials:
[1] SUNOVION SUBMITS NEW DRUG APPLICATION TO THE FDA FOR APOMORPHINE SUBLINGUAL FILM (APL-130277) FOR THE TREATMENT OF OFF EPISODES ASSOCIATED WITH PARKINSON'S DISEASE
[2] Sunovion Submits NDA for Sublingual Parkinson's Treatment
[3] Allergan and Richter Announce Positive Topline Results from Third of Three Pivotal Trials of Cariprazine in Bipolar I Depression
[4] Denali-WashU team clears Alzheimer's plaques in mice by targeting a faulty APOE gene
Original Title: Recent Research Progress in the Field of Central Nervous System Diseases (No. 52)
We are a professional Chinese supplier of Natural Antioxidants; we supply various products of Plant Antioxidants; and we can providing product images and basic parameters with each Natural Oxidants and Anti-Free Radical Ingredients, such as Astaxanthin Powder, OPC 95%, Turmeric Root Extract, Lycopene Powder, Beta-carotene Powder, etc. Look forward to your cooperation!
Astaxanthin Powder,OPC 95%,Turmeric Root Extract,Lycopene Powder,Beta-carotene Powder
Xi'an Quanao Biotech Co., Ltd. , https://www.quanaobiotech.com