It's time to pay more attention! China's chromatography "core" of Protein A affinity filler domestic replacement
Guide
Biologics represented by monoclonal antibodies are undoubtedly one of the most eye-catching focuses in the pharmaceutical industry. Take Trepril monoclonal antibody as the only listed domestic melanoma PD-1 product, which was opened at the end of February 2019. Since the first prescription, its good market performance has provided a strong guarantee for the performance of Junshi Bio. CICC analysts believe that Treipoli is expected to continue to expand its market share in 19 years, and it is expected that the annual sales will exceed 600 million. yuan!
2019 global TOP 10 drug sales forecast (100 million US dollars)
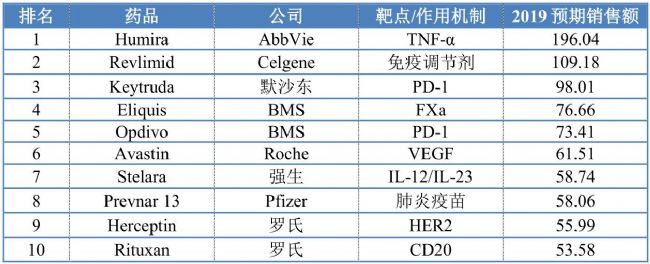
Source: EvaluatePharma
In addition, between 2012 and 2019, six heavyweight monoclonal antibody patents have expired one after another, opening the door for biosimilar drugs. The global biosimilar drug market is expected to exceed $30 billion in three years.
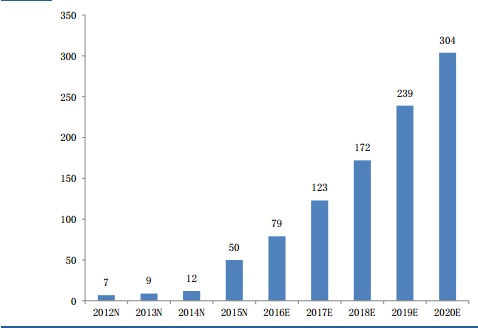
Global biosimilar drug market size (unit: billion US dollars)
The market for monoclonal antibodies is so delicate, and it is not difficult to find out the status quo of its R&D and production. In today's price war in the field of antibodies, its upstream research and development technology is developing rapidly, and the requirements for downstream output and production efficiency are also increasing. It can be said that the industry has always had a high standard of demand for cost-effective chromatographic packing. Although there are many excellent chromatographic filler products for pharmaceutical companies to choose from abroad, in addition to the high price, the actual situation of the “ban†card neck has recently been encountered, and more and more enterprises have realized that this is the chromatographic packing. In the field of “Chromatography Chipsâ€, there is an urgent need for high-quality domestically produced alternative products to break the extreme passive situation caused by foreign monopoly. In this respect, Nanotech has provided us with mature and excellent solutions, among which UniMab and NanoMab Protein A affinity fillers are Very excellent illustration.
Screening for key assessment elements for the purchase of Protein A affinity fillers
Monodisperse matrix microspheres: Protein A affinity fillers are expensive and belong to the “noble choice†in chromatographic purification media. The existing Protein A affinity fillers are basically based on polydisperse systems, which is not conducive to better cooperation. A affinity advantage, monodisperse system can bring better packing experience, good mass transfer effect, concentrated concentration, lower back pressure and many other significant advantages;
High load: high load can ensure more efficient purification production in the same time. If high dynamic binding capacity can be maintained at higher flow rate, it is more conducive to balance production efficiency and production;
Long life: The cost of chromatographic medium is generally high. The length of use will affect the economics of purification to a certain extent, and it can also avoid the potential problems caused by replacing fillers to some extent.
High alkali resistance: It can withstand 0.5 M NaOH solution to effectively control bioburden and prevent potential pollution. After 100 cycles of cleaning, the dynamic load should be maintained above 95%.
High mechanical strength: high mechanical strength ensures that the chromatographic medium can withstand higher flow rates and column pressures. High-concentration samples can be loaded directly, with wider production conditions, higher production efficiency, and can be filled in process amplification and production. Higher column height, effectively increasing single-batch capacity, reducing the number of batches and the number of inspections by QC and other departments, ensuring higher productivity and enterprise efficiency;
The purification repeat stability is good: the stability of the chromatographic medium between batches is high, which can ensure the process requirements and purification efficiency of the purification production, greatly reduce the interference of the variable factors in the purification production, and save manpower and time cost.
Technical service is timely and convenient: to teach people to fish and to teach people to fish, high-quality fillers with professional and timely separation and purification technology services to achieve the best results for all-round customer service, maximize customer purification experience, timely resolution of separation Problems in purification, efficient and professional;
The supply chain is safe and reliable: in addition to the stable supply capacity and capacity, the supplier's qualifications and standards are up to standard, it is also necessary to ensure that the chromatographic medium will not be out of service or banned due to external reasons.
It should be said that the development and production technology of chromatographic media has been monopolized in the hands of foreigners for a long time. This monopolistic operation is not a national academic paper published in the field of chromatography or several breakthroughs can be reversed, even though It has been reported that China's papers in the field of chromatographic purification have already ranked first in the world. In recent years, we have repeatedly reported that the Western world has blocked us from the high-tech fields such as chips, and more and more companies are beginning to realize the importance of mastering the core technologies that can be controlled independently. Sex: In the field of chromatography, Nawei Technology is one such enterprise. Let's take the Protein A affinity filler as an example to see what excellent characteristics of our high-tech domestic fillers can meet the above key assessment factors. In this case, an independent and controllable alternative to expensive foreign monopoly products.
Monodisperse UniMab® Features 1: High mechanical strength and high dynamic load
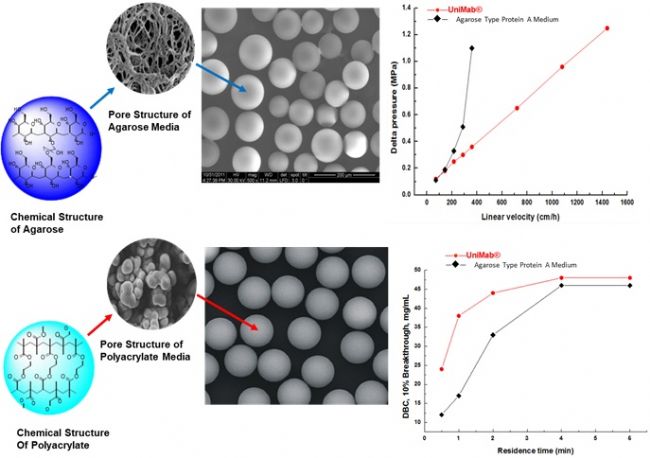
As shown above, the linear relationship between the pressure flow rate of the monodisperse UniMab chromatography medium and the polydisperse soft matrix Protein A affinity chromatography medium shows that the UniMab has better mechanical strength, and the high mechanical strength can bring the following advantages in use:
1 Improve production efficiency and flow rate, and shorten the purification cycle;
2 It can be loaded with long columns to increase the batch processing capacity and process more samples in a shorter time;
3 Refillable column to extend service life;
4 directly on high concentration, high viscosity samples;
5 Reduce debris to avoid clogging of the screen and reduce pressure;
6 wider operating space.
In addition, the above figure also compares the DBC (Dynamic Binding Capacity) values ​​of the two fillers at different retention times. It can be clearly seen that UniMab has a higher dynamic load on the target protein at the same retention time due to chromatography. Both are carried out at a certain flow rate, so the dynamic loading of the packing is more effectively used to guide the determination of the loading. As can be seen from the figure, although the two chromatographic media are similarly downloaded at low flow rates, the UniMab loading is much higher than the Agarose Type Protein A Resin at high flow rates. Because monodisperse UniMabs have uniform particle size, good channel permeability, and fast binding of antibodies to Protein A ligands, UniMab has higher loading at high flow rates (short column retention times), which will be very beneficial. Customers improve production efficiency and reduce production costs.
Monodisperse UniMab® Feature 2: Dynamic Load Throughput Graph
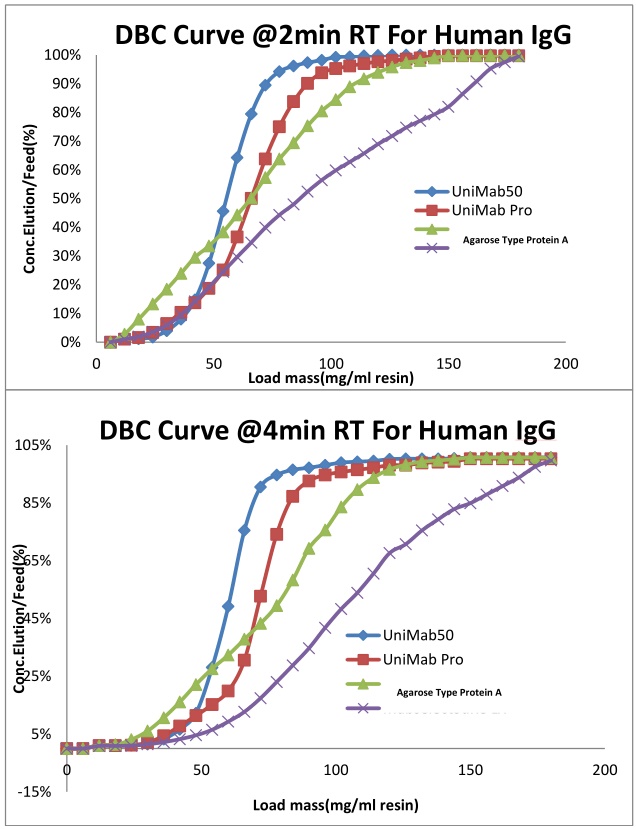
Compared with the soft gel matrix Protein A affinity chromatography medium, the nano-micro UniMab has a steeper dynamic loading curve indicating that the pore structure of the nano-filler is more conducive to the rapid mass transfer of the antibody.
Monodisperse UniMab® Features 3: Wait for retention time to zoom in and hold a higher bed
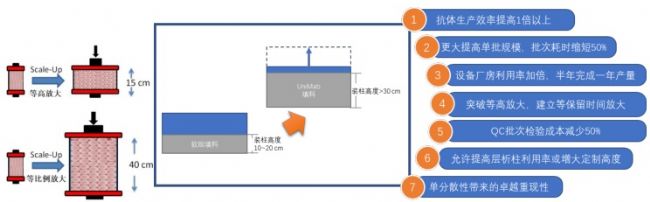
The high mechanical strength allows UniMab to be loaded with higher column beds to increase batch throughput and reduce buffer usage, resulting in increased productivity, reduced production costs and energy consumption.
For the UniMab chromatography medium, the process is amplified by the same retention time. For details, please refer to the article “Improve the purification yield of monoclonal antibody, only the magnification is amplified†published by the public on January 5, 2018, or directly call the hotline. We communicate.
Monodisperse UniMab® Features 4: Stable ligand bonding, less recycling
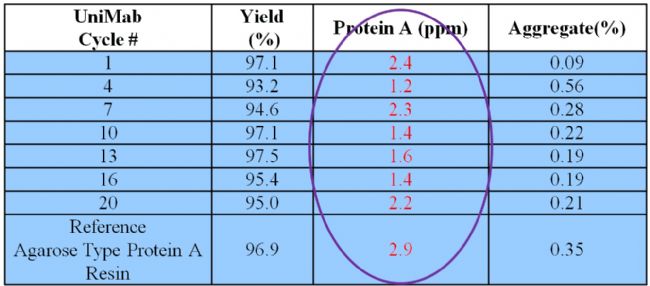
After multiple cycles of Protein A affinity chromatography media for antibody purification, if the binding of Protein A on the chromatographic medium is less, the stability of the matrix and ligand bonding of the chromatography media is better. The quality and purity of the antibodies is very important. From the experimental data in the above table, the stability of the ligand of the UniMab Protein A affinity chromatography medium is significantly better than that of the soft matrix matrix.
Monodisperse UniMab® Features 5: Long life and low production costs
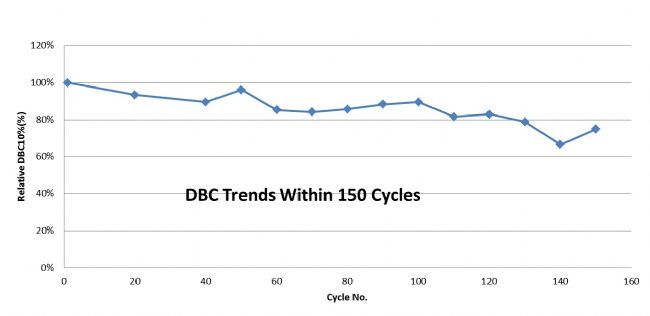
As shown in the figure, the UniMab Protein A affinity chromatography medium can maintain a dynamic binding capacity of about 90% after 100 cycles of 10% flow through. This not only can significantly reduce the production cost of the customer, but also better confirms that the ligand binding of the UniMab affinity filler is stable and the shedding is extremely low, which can better ensure the stable and orderly purification production.
In addition, Nano's new UniMab Pro Protein A affinity chromatography media is a fully upgraded version that excels in terms of lifetime and has a picture of the truth!

Monodisperse UniMab® Feature 6: Good chromatographic reproducibility and high purification stability
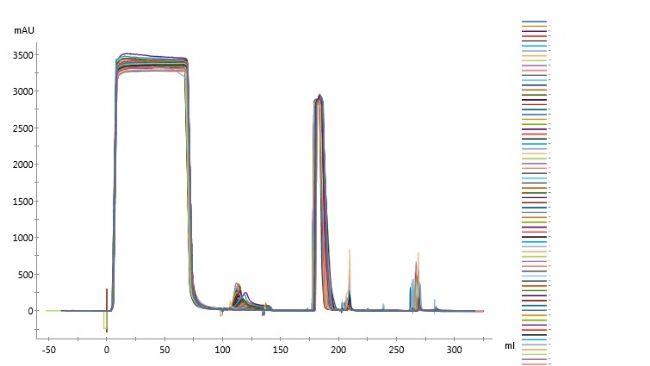
The above figure is a preparative chromatogram after purification of the antibody with UniMab filler for 150 times. It can be seen intuitively that the multiple separation and purification of the map can achieve complete coincidence, and the chromatographic reproducibility is very good, which can not only meet the research and development production, etc. The process requirements and purification efficiency of the link, and greatly reduce the interference of variable factors in the purification production, saving manpower and time costs.
Monodisperse UniMab® Feature 7: Increase Productivity and Economics
UniMab is based on monodisperse polymethacrylate porous microspheres, which has high particle size uniformity and good channel permeability, so the antibody binds fast with the Protein A ligand. UniMab has a higher loading capacity at high flow rates (short column retention time), so it can be used to scale up the process to increase the throughput and productivity of the antibody. Previous studies have said that when Protein A is reused more than 30 times, the mobile phase cost will gradually increase higher than the cost of Protein A medium, and UniMab can significantly reduce mobile phase consumption, thereby reducing production costs and environmental costs of waste disposal. .
In summary, the monodisperse UniMab Protein A affinity chromatography medium not only has high separation efficiency for antibodies, but also can obtain high purity antibodies with high recovery rate, and can also significantly reduce production costs including buffer dosage, manpower, time, and the like. It has become one of the first choices for the separation and purification of biological macromolecules such as antibodies and recombinant proteins.
Nano-Protein A affinity chromatography medium: the leading choice for domestic high-tech chromatography packing innovation
Today, domestic companies generally recognize that we should use technology innovation and industrialization development to quickly break through many key technical fields of foreign monopoly, and firmly grasp the initiative and enterprise lifeline of high-tech self-control in our own hands. Solve the problem of "card neck" once and for all. Nano Micro Technology's more than ten years of scientific research and independent innovation has finally made its own invisible champion in the precision manufacturing of nano microspheres. It should be an achievement worthy of pride and pride of the people, and the current continuous stream chromatography new technology. It is increasingly favored by domestic and foreign companies, and the nano-monodisperse series of chromatographic packings are very suitable for this new technology, far superior to the traditional polydisperse soft rubber series, so it seems that we have not only achieved foreign Breakthroughs in the monopoly field have even achieved catch-up development in the application of new technologies.
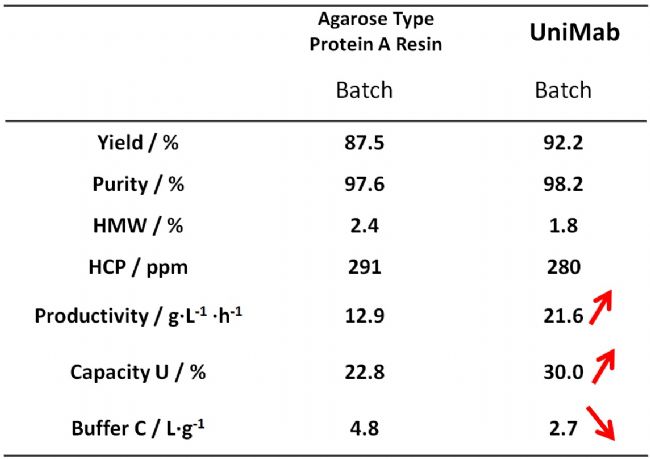
Regarding the specific application performance of nano-micro UniMab affinity filler in two-column continuous flow chromatography, Professor Lin Dongqiang from the College of Chemical Engineering and Bioengineering of Zhejiang University also conducted a special comparison with MabSelect SuRe affinity filler of internationally renowned GE. The research and research results were published in the Chinese core journal "Journal of Chemical Engineering of Colleges and Universities", and the paper was entitled "Process Design and Application of Affinity Separation of Antibodies by Two-column Continuous Flow Chromatography". Professor Lin's research team affirmed the outstanding performance of UniMab affinity filler through careful and comprehensive all-round research and data analysis. It can be said that it provides another proof for the domestic substitution of Chinese chromatographic "core". In this article, I attached a preview of the page of this article. Interested readers can click on the picture of the paper to read the full text.
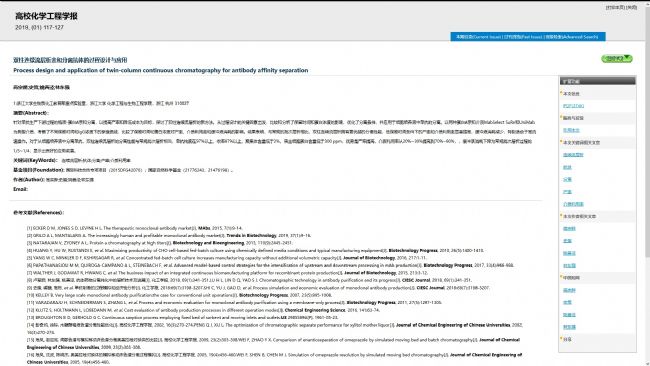
Advantages of nano-Protein A affinity chromatography media:
High mechanical strength, high dynamic load at high flow rates, can greatly improve production efficiency
Good alkali resistance, can meet CIP & SIP requirements
Long service life and low production costs
Significantly reduced buffer usage, further reducing production costs
High recovery rate, high purity of target antibody, low separation of HCP and loss of Protein A ligand
The world's first mass-produced monodisperse Protein A affinity chromatography media, ideal for continuous chromatography media
Nano offers a complete solution for cost-effective antibody purification
(Welcome to contact us directly for details)
Fire Extinguisher Ball,Elide Fire Ball,Afo Fire Extinguisher Ball,Elide Fire Extinguisher Ball
DONGGUAN TENYU TECH.INC , https://www.tenyutech.com