With the announcement of Kite Pharma, the marketing authorization application (MAA) using its CAR-T cell product (axicabtagene ciloleucel, KTE-C19) has been submitted to the European Food and Drug Administration (EMA); Novartis's CAR-T therapy CTL019 (tisagenlecleucel- T) A full vote recommendation from an advisory board expert was obtained with a 10:0 vote. In decades of tortuous research, CAR-T cell therapy has also ushered in a phased victory.

CAR-T cell therapy (image source EMBO)
Completed and ongoing CAR-T cell clinical trials
As of the end of 2016, 220 CAR-T cell trials have been recorded, including 188 ongoing clinical trials including 9 long-term follow-up studies. Most of the clinical trials conducted were mainly to evaluate the safety and dose standard of Phase 1 (128), but the evaluation of the efficacy of clinical trials of Phase 1/2 and Phase 2 is gradually catching up, especially the CAR-targeted CAR- T cell therapy progresses fastest (75 of phase 1/2 or phase 2 trials include 39)
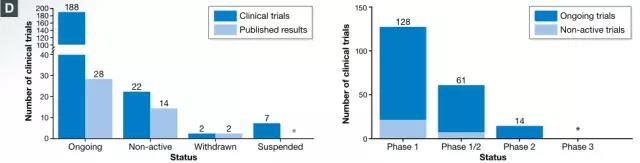
Test status of published CAR-T cell therapy registered with ClinicalTrials.gov, including long-term follow-up studies (image source EMBO)
In the first-generation CAR-T cell trial, researchers treated patients with advanced epithelial ovarian cancer or metastatic renal cell carcinoma by targeting folate receptor or carbonic anhydrase IX (CAIX) (Kershaw et al., 2006; Lamers et al., 2006). ). The next two registered clinical trials reported that one patient with neuroblastoma and one patient with follicular lymphoma achieved complete remission (Park et al., 2007; Till et al., 2008).
In recent years, with the major breakthrough of CD19-specific CAR-T cells in the treatment of B-cell malignancies, clinical studies have shown that remission is no longer directed at individuals, but the vast majority of patients. Based on promising results, the number of clinical trials of CAR-T cell therapy has begun to increase substantially, exponentially. In 2016 alone, 62 new CAR-T cell clinical trials were registered on ClinicalTrials.gov.

Timeline for CAR-T cell trial (image source EMBO)
CAR-T cell therapy originally originated in the United States and then spread to other parts of the world. Currently, 89 CAR-T cell clinical trials are being conducted outside the United States, with the largest number of clinical trials in China (66) and 14 in Europe (8 in the UK, 3 in Germany, 3 in France). Compared with the United States and China, Europe is clearly a backward side.
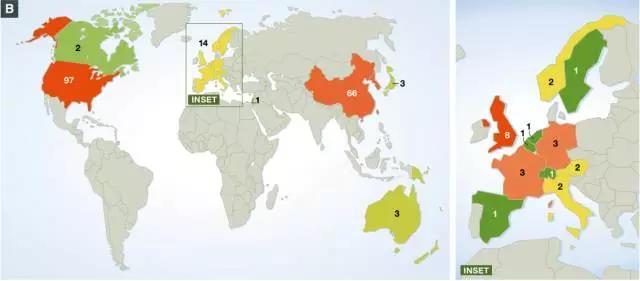
Geographical distribution of ongoing CAR-T cell clinical trials worldwide (image source EMBO)
The current trial included 133 for hematologic malignancies and 78 for solid tumors. For tumors of the hematopoietic and lymphatic systems, there are 17 different CAR antigens being studied. The most commonly used antigen is CD19 (56 in a new type of clinical trial, 8 stability tests), and the study on solid tumors is based on targeting CD22 antigen . Previous studies have focused on the treatment of colorectal cancer, breast cancer, gastric cancer, adenocarcinoma, and secondary liver cancer with CEA as a targeted antigen. Targets currently under trial also include mesothelin, ErbB2 / Her2, GD2 (neuroblastoma or sarcoma) or GPC3 (hepatocellular carcinoma).
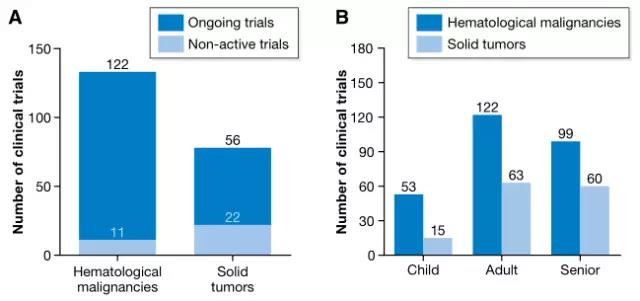
Number of clinical trials of hematological malignancies and solid tumors (Source EMBO)
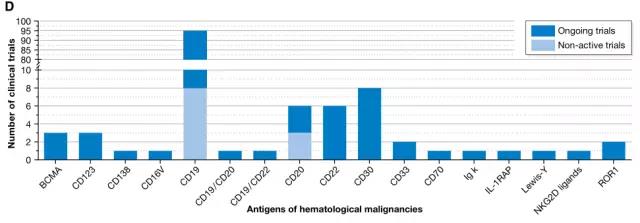
17 targeted antigens for hematological malignancies (Source EMBO)
Most clinical trials use autologous cell therapy, unselected PBMC (peripheral blood mononuclear cells) as a starting material, which produces CD4 and CD8 T cells with an activated effector T cell phenotype under the classical stimulating factor IL-2. CAR-T cell product. In recent years, methods for isolating defined T cell subsets or transforming T cells into a certain phenotype have been developed (Xu et al, 2014; Ramos et al, 2016; Turtle et al, 2016).
In addition, automated manufacturing may be an option to simplify the process and enhance CAR-T cell production. CD19-CAR-T cells generated using automated GMP cell processing systems have been shown to have transduction efficiency, phenotype, efficacy and overall yield. Comparison with CD19-CAR-T cells produced by conventional techniques (Mock et al, 2016; Priesner et al, 2016).
In general, CAR-T cells are injected intravenously. However, other studies have tried other modes of administration: intratumor (You et al, 2016, intracranial (Brown et al, 2015) or intraperitoneal injection (Koneru et al, 2015), hepatic artery (Katz et al, 2015), intrathoracic perfusion ( Petrausch et al, 2012). To increase the tolerance of treatment and reduce the risk of side effects, a given CAR-T cell dose is usually divided into multiple injections (eg, 3 injections per day).
It is worth noting that the total number of cells injected depends on the percentage of CAR-positive T cells within the product and is highly variable between studies and in separate trials.
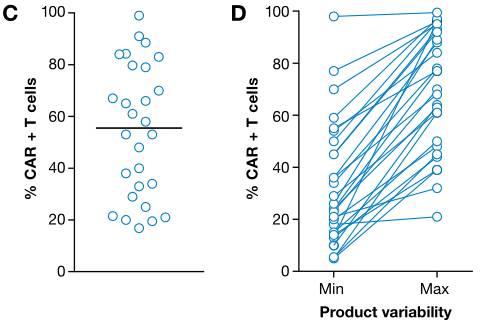
Percentage of CAR-positive cells in CAR-T cell products (Image source EMBO)
For four generations of CAR-T cells, a slight preference for the use of gamma-retroviral vectors (RV) directly associated with lentiviral vectors (LVs) was observed. Only a few clinical studies have used electroporation to transfer CAR constructs. In most trials, second-generation CARs have been transformed into clinical applications, and third- or fourth-generation CARs targeting CD19 are being tested.

Number of clinical trials of four generations of CAR-T cell therapy (image source EMBO)
Clinical Benefits of CAR-T Therapy for Cancer Patients
The most famous case for patients benefiting from CAR-T cell therapy may be Emily Whitehead. As a child with relapsed acute lymphocytic leukemia (ALL), Emily has lived healthily for 5 years (http ://emilywhitehead.com). NCT01626495 is the clinical trial in which Emily is involved.
For B-cell malignancies, CAR-T cell therapy appears to be particularly effective. The reason is because of the selective and uniform expression of tumor cells of CD19 or CD20, and it is easy for CAR-T cells to recognize them.
In a clinical trial of CD19 CAR-T cell therapy, the objective response rate was over 60% in 243 patients (199 adults, 44 children) who received treatment, and only 20% of patients did not respond. It is worth noting that clinical trials appear to be age-independent in trials involving children and adults (Cruz et al., 2013; Maude et al., 2014; Lee et al., 2015; Zhang et al., 2016).
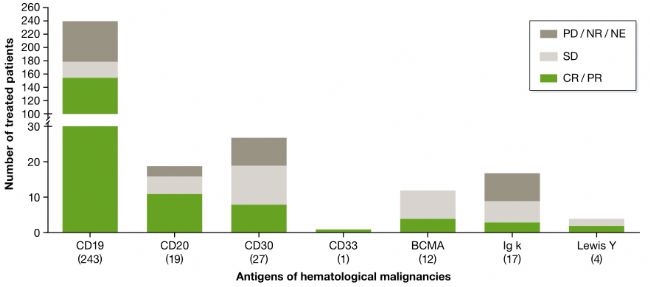
The clinical outcome of CAR-T in the treatment of hematologic malignancies, the number of patients treated is located in parentheses below the target antigen (image source EMBO)
In five clinical trials numbered NCT00968760, NCT01865617, NCT01815749, NCT01626495, NCT01044069, more than 85% of patients achieved the best clinical outcome - complete remission (CR). In these trials, the time points for the assessment ranged from 4 weeks (Turtle et al., 2016) to 30 months (Kebriaei et al., 2016).
Patients enrolled included different levels of detectable disease in the bone marrow (BM), extramedullary or cerebrospinal fluid - acute lymphoblastic leukemia (ALL) or non-Hodgkin's lymphoma (NHL). Also included are minimal residual disease (MRD) or morphological disease patients defined by the percentage of mother cells in the BM.
Interestingly, there appears to be no significant difference in response rates between patients with morphological disease and MRD (Davila et al., 2014; Maude et al., 2014; Turtle et al., 2016; Park et al., 2017). However, after 18 months of follow-up, MRD patients have significantly longer lifespans (Park et al., 2017). Therefore, a low level of tumor burden appears to increase the durability of CAR T cell therapy, at least in the trials of treating ALL.
Therefore, it is feasible to reduce the tumor burden by chemotherapy before CAR-T cell therapy. Moreover, after receiving CAR-T cell therapy, patients with complete remission can receive treatment with allogeneic hematopoietic stem cell transplantation (HSCT) to provide additional therapeutic potential.
In the study of acute lymphoblastic (ALL), CAR-T cell-induced remission has been consolidated to varying degrees by transplantation [n=3/30, 11%; (Maude et al., 2014); n=7/14, 50% (Davila et al., 2014); n=10/14, 71% (Lee et al., 2015); n=13/27, 48% (Turtle et al., 2016)]. Despite some differences and limitations of short-term follow-up, the persistence of responses between studies appears to be very similar. This suggests that CAR-T cell therapy may provide significant clinical efficacy regardless of whether the patient is receiving HSCT.
In addition, it is often overlooked in the same study that most patients underwent transplantation before receiving CAR-T cell therapy, which indicates that tumor-insensitive engineered CAR-T cells are insensitive to graft-versus-leukemia effects. It is sensitive.

James N. Kochenderfer, MD (image source ccr.cancer.gov)
Overall, CD19 CAR-T cell therapy is most effective in patients with acute lymphoblastic (ALL), followed by non-Hodgkin's lymphoma (NHL) and chronic lymphocytic leukemia (CLL), which also indicates CAR-T cell therapy. The role played in different cancer types is different.
In addition, the initial clinical outcome of CAR-T in the treatment of NHL is also very exciting. Of the nine high-grade chemotherapy-resistant NHL cases, four patients achieved complete remission and the other two had partial remission (Kochenderfer et al., 2015). ). Preliminary results from subsequent trials sponsored by Kite Pharma and the same CAR T cell product confirmed these results, with four of the seven patients achieving complete remission for 12 months (Locke et al., 2017).
However, the general high-grade chemotherapy-resistant NHL has a poor prognosis, and the median survival time is only a few weeks. Therefore, this clinical result also prompted Kite Pharma to submit a marketing application for CAR-T cell product (KTE-C19) to the FDA. . Compared to CD19, the clinical efficacy of other tumor antigens targeting hematologic malignancies is less pronounced, although most ongoing trials have not yet reached the end.
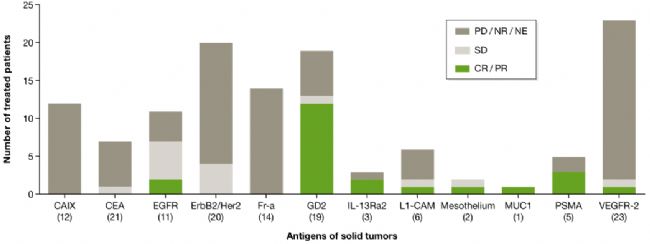
The clinical outcome of CAR-T in the treatment of solid tumors, the number of patients treated is located in parentheses below the target antigen (image source EMBO)
Inconsistent with the results of hematological malignancies, CAR-T treatment of solid tumors (12 different antigen targets) is not as smooth, but in a phase I clinical trial of patients with neuroblastoma, Targeted CAR-T cell therapy with GD2 resulted in a patient's complete response rate (CR) of more than 50%. (Louis et al., 2011).
Conclusion

Recently, Kite Pharma announced that it has submitted to the European Food and Drug Administration (EMA) the use of axicabtagene ciloleucel (KTE-C19) for the treatment of refractory diffuse large B-cell lymphoma (DLBCL), transformed follicular lymphoma (TFL), and primary Marketing Authorization Application (MAA) for mediastinal B-cell lymphoma (PMBCL) for cancer patients who are not eligible for autologous stem cell transplantation. This application is the first chimeric antigen receptor T cell (CAR-T) therapy proposed to EMA. Novartis's CAR-T therapy CTL019 (tisagenlecleucel-T) was also recommended by the advisory committee experts with a 10:0 vote .
The first batch of CAR-T cell products will also be ushered in the US and European markets. However, due to the unique design, delivery, regulation, and genome editing of CAR to insert unlimited selection of CAR genes, we can easily imagine that more CAR-T cell-based products will enter clinical research in the future, and this will Increase the choice of treating solid tumors and simplify the production process of CAR-T cells.
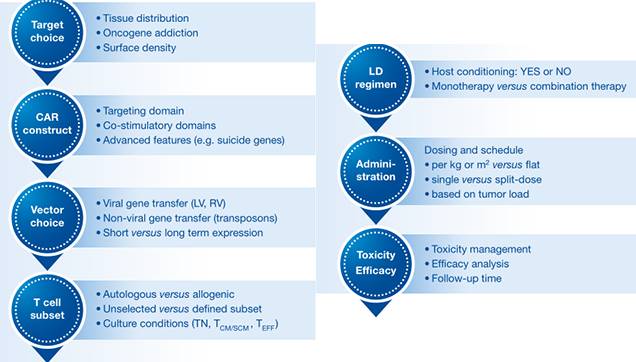
Important Drivers in CAR-T Cell Trials (Image Source EMBO)
Researchers need to get as much information as possible from the trials completed to date, which will help these new initiatives, although CAR-T cell therapy (CAR construction, manufacturing processes, indications and clinical trial design) is too complex for A detailed comparison of the results of different trials seems unlikely, but some of the important factors common to all types of CAR-T cell therapies can be drawn from clinical data.
Finally, it must be emphasized that it is also important to develop a toxicity management plan and identify biomarkers to predict common toxicity, as the final overall survival data will allow for a true comparison of the long-term benefit risk outcomes of CAR-T cell therapy.
Reference source:
DOI 10.15252/emmm.201607485
Http://
Https://
Foot Spa Machine,Foot Tub Spa Pedicure,Foot Bath Massager,Bubble Foot Spa
Huaian Mimir Electric Appliance Co., LTD , https://www.footspamachine.com